ÁREA
Química Medicinal
Autores
dos Santos Filho, J.M. (UFPE) ; da Silva Júnior, G.R.P. (IFPE)
RESUMO
N-acylhydrazone (NAH) has been recognized as a versatile and promising leitmotiv in medicinal chemistry due to the pharmacophoric –CO–NH–N= group and the different substituents, leading to a large diversity of structures. NAH derivatives are largely investigated for their biological activities, and some of them have attained preclinical and clinical trials, while others are approved drugs. The design and synthesis of a plethora of molecules bearing the NAH scaffold lead to the report of various pharmacological activities, including antitumor, antimicrobial, antiviral, antiparasitic, anti- inflammatory, immunomodulatory, and antiprotozoal. Therefore, a series of N-piperidinyl-N-acetohydrazones was designed and synthesized as potential antitumor agents.
Palavras Chaves
N-Piperidinylhydrazide; N-Acylhydrazones; Antitumor activity
Introdução
The N-acylhydrazone (NAH) moiety is considered a privileged structure in medicinal chemistry, since it can be recognized by many distinct biological receptors, leading to a broad range of pharmacological activities (THOTA et al, 2018). In fact, several reports have demonstrated the importance of N-acylhydrazone derivatives as antitubercular, antiviral, analgesic and anti-inflammatory, anticonvulsant, antitrypanosomal, antipsychotic, and vasodilatory agents, amongst others (SOCEA et al, 2022) (Figure 1). NAH scaffold is an important pharmacophoric group for the development of novel biologically active molecules, several of them emerging as lead compounds to the descovery of new drugs. Efforts based on the NAH structure has led to approved drugs available in the pharmacopeia, e.g. nitrofurazone, nitrofurantoin and carbazochrome (MAIA et al, 2014). Additionally, several NAH-bearing compounds are in clinical trials for their biological responses, such as aldoxorubicin, an antitumor agent, currently in phase III trials (MAIA et al, 2014). The NAH motif is particularly promising as a base for the synthesis of a plethora of potent anticancer agents acting via different mechanisms. Due to their structural diversity, the design of novel NAH derivatives is especially easy and their synthesis is potentially viable (KASSAB, 2023). Starting from this perspective, the Laboratory of Design and Synthesis Applied to Medicinal Chemistry-SintMed has planned and prepared a new series of N-piperidinyl- N-acetohydrazones (Scheme 1), exploiting some aspects of their structures that can lead to the discussion of their structure–activity relationship (SAR) and their potential anticancer responses.
Material e métodos
Reactions’ progress was monitored by thin-layer chromatography (TLC), performed onto glass-backed plates of silica gel 60 PF254 with gypsum from Merck, and all compounds were detected by ultraviolet light (254 nm) and iodine. Melting points were determined with a capillary apparatus and were uncorrected. IR spectra were recorded on a Tensor27 FTIR spectrometer from Bruker with the samples being analyzed as KBr pellets. The synthetical route is depicted in Scheme 1. The alkylation of piperidine (1) was achieved using ethyl chloroacetate (2) in acetonitrile at room temperature overnight through a substitution nucleophilic bimolecular (SN2) in the presence of potassium carbonate as a base, giving the ethyl N-piperidinyl acetate (3). Compound (3) was isolated after workup in a two-step extraction, one with 10% HCl solution and dichloromethane, and the second extraction was carried out in a basic medium after neutralization with 20% Na2CO3 solution. The final organic layer containing the product was distilled under a vacuum to furnish the crude product. The key intermediate N-piperidinyl acetohydrazide (4) was obtained after refluxing (3) in the presence of hydrazine hydrate overnight and isolation. A neat equimolar mixture of (4) and appropriate aryl aldehydes (5a-e), catalyzed by 10% mol CeCl3•H2O, was heated at 65 ºC to the melting for 60 minutes, so derivatives N- piperidinyl-N-cetohydrazones (6a-e) were observed by TLC and then isolated by column chromatography using AcOEt/Haxanes as eluent.
Resultado e discussão
The proposed methodology (Scheme 1) started with the reaction between piperidine
(1) and ethyl chloroacetate (2) via substitution nucleophilic bimolecular
(SN2) in the presence of potassium carbonate as base leading to ethyl
N-piperidinyl acetate (3) as a pale yellow liquid in 81% yield, which was
used without further purification. Refluxing (3) with hydrazine hydrate 55%, the
key intermediate N-piperidinyl acetohydrazide (4) was isolated in 92%
yield as a white solid after extraction and solvent removal. A neat equimolar
mixture of (4) and appropriate aryl aldehydes (5a-e), catalyzed
by 10% CeCl3•H2O, was heated at 65 ºC to the melting for
60 minutes. The reaction was monitored by TLC before its conclusion. The
reaction mixture was purified by column chromatography using AcOEt/Hexanes as
eluent. The target N-piperidinyl-N-acetohydrazones (6a-e) were
isolated as pure solids, whose characterization was carried out by infrared (IR)
spectroscopy, measured in cm–1. Analytical results are disclosed as
follows.
6a: Rf 0.52 (AcOEt), yellow solid, yield 75%, mp 160-163 °C,
IR: 3320 (N-H), 2922 (C-H), 1680 (C=O);
6b: Rf 0.39 (AcOEt), orange solid, yield 66%, mp 153-155 °C,
IR: 3326 (N-H), 2921 (C-H), 1696 (C=O);
6c: Rf 0.51 (AcOEt/MeOH 9:1), yellow solid, yield 85%, mp 197-
198 °C, IR: 3258 (N-H), 2934 (C-H), 1698 (C=O);
6d: Rf 0.54 (AcOEt/MeOH 9:1), white solid, yield 80%, mp 202-
204°C, IR: 3188 (O-H, N-H,), 2970, 2830 (C-H), 1662 (C=O);
6e: Rf 0.35 (AcOEt/MeOH 9:1), white solid, yield 75%, mp 173-
174°C, IR: 2940 (C-H), 1690 (C=O).
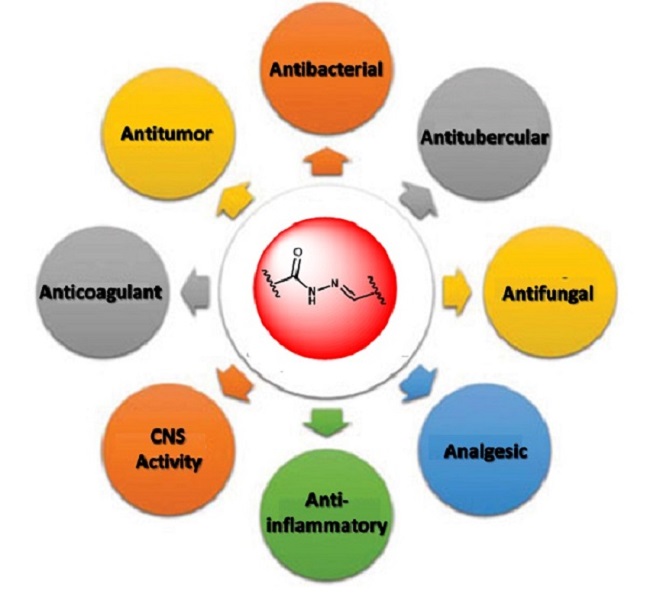
Figure 1. Biological activities of N-acylhydrazones
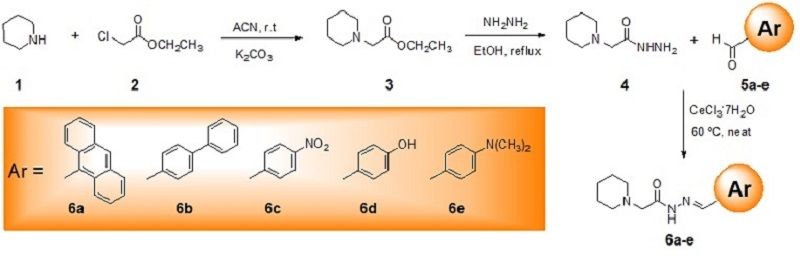
Scheme 1. Synthetic route for 6a-e
Conclusões
In this work a successful methodology for the synthesis of 5 N- piperidinyl-N-acetohydrazones was investigated. This series of molecules is of synthetic and biological interest as anticancer agents. Further studies on their biological activities are a promising field for the comprehension of their structure-activity relationships (SAR), especially due to the possible molecular diversity that can be exploited starting from this preliminary group of molecules.
Agradecimentos
The authors are grateful to the Analytical Centre of Fundamental Chemistry Department, Universidade Federal de Pernambuco, for the spectroscopic experiments.
Referências
KASSAB, A.E. Anticancer agents incorporating the N-acylhydrazone scaffold: Progress from 2017 to present, Arch. Pharm. 356(5), 2023.
MAIA, R.D.; TESCH, R.; FRAGA, C.A.M. Acylhydrazone Derivatives: A Patent Review. Expert Opin. Ther. Pat. 24, 1161–1170, 2014.
SOCEA, L.I.; BARBUCEANU, S.F.; PAHONTU, E.M.; DUMITRU, A.C.; NITULESCU, G.M.; SFETEA, R.C.; APOSTOL, T.V. Acylhydrazones and Their Biological Activity: A Review. Molecules, 27(24), 8719, 2022.
THOTA, S.; RODRIGUES, D.A.; PINHEIRO, P.S.M.; LIMA, L.M., FRAGA, C.A.M.; BARREIRO, E.J. N-Acylhydrazones as drugs. Bioorg. Medic. Chemistry Letters, 28(17), 2797–2806, 2018.