ÁREA
Química Orgânica
Autores
dos Santos Filho, J.M. (UFPE) ; da Silva Júnior, G.R.P. (IFPE)
RESUMO
There are numerous methods for the N-alkylation of cyclic secondary amines. Traditionally, alkylation has been performed using alkyl halides in organic solvents in the presence of a base, such as metal amides, sodium hydride, and potassium hydroxide under dry solvent conditions. However, these methods have the drawback of using strong metallic bases, which cause hydrolysis of ester groups, limiting their application to the synthesis of N- cycloamine esters, which are important building blocks for the synthesis of several useful molecules. The use of potassium carbonate under mild conditions leads to excellent results, allowing the development of a standard methodology for the synthesis of ethyl N-cycloamine acetates and the measurement of their boiling points.
Palavras Chaves
N-cycloamine acetates; N-alkylation; Ester conversions
Introdução
Biogenic amines are ubiquitous in animals, plants, microorganisms, and humans. They have several critical biological roles in the body and are essential for physiological functions such as the regulation of growth and blood pressure and control of nerve conduction. Besides, they are required in the immunologic system of intestines and in maintaining the activity of the standard metabolic functions, as well as in the respiratory, cardiovascular systems, and allergic reactions (ERDAG et al., 2018). It was ascertained that synthetic amines can be recognized by proteins and enzymes, leading to biological responses in living beings. Hence, alkylated N-cycloamines are of importance in medicinal chemistry due to their central role in biological responses, since they are similar to some natural amines such as histamine, tryptophan, serotonin, and the large class of alkaloids (HAMEED et al., 2017). An interesting molecular design strategy is molecular hybridization, which builds a structure bearing two or more pharmacophores in one molecule. The synthesis of N-alkylated cycloamine esters is of high interest because the ester structure is a building block for a plethora of new molecules, as seen in Figure 1 (CHEMINFOGRAPHIC, 2022). The alkylation of N-clycloamines is a process that can be carried out using several methodologies (MESHRAM et al., 2009), but the presence of ester groups demands suitable methodologies with this chemical functionality. Therefore, the optimization of a method for the synthesis of 9 ethyl N- cycloamine acetates based on the literature (SALEH et al., 2017) was the target of this work as well as the determination of their physical properties, which are not standardized until now.
Material e métodos
Reactions’ progress was monitored by thin-layer chromatography (TLC), performed onto glass-backed plates of silica gel 60 PF254 with gypsum from Merck, and all compounds were detected by ultraviolet light (254 nm) and iodine. Boiling points were determined using a kugelrohr apparatus from Büchi. IR spectra were recorded on a Tensor27 FTIR spectrometer from Bruker with the samples being analyzed as KBr pellets. The synthetical route is depicted in Scheme 1 and starts with the alkylation of cycloamines (1a-i) using ethyl chloroacetate (2) in acetonitrile at room temperature overnight through a substitution nucleophilic bimolecular (SN2) in the presence of potassium carbonate as base. The ethyl N-cycloamine acetates (3a-i) were isolated after workup using a two-step extraction, one with 10% HCl solution and dichloromethane used to remove organic impurities. The second extraction was carried out in a basic medium after neutralization with 20% Na2CO3 solution. The final organic solution containing the product was distilled under a vacuum to furnish the crude products as yellow liquids, which were distilled at 18 mbar at a kugelrohr apparatus.
Resultado e discussão
Following the proposed methodology (Scheme 1), ethyl chloroacetate (2) was used
in the synthesis in excess (1.2 eq.) and the TLC control showed the total
consumption of the N-cycloamines (1a-i). To remove the excess of
(2), the products were extracted with 10% HCl and dichloromethane. Next, the
acid aqueous solution was neutralized with 20% Na2CO3 and
extracted with dichloromethane. After removing the organic solvent, crude
products were isolated as yellow liquids, except compound (3f), a white solid.
All liquid compounds were distilled at a kugelrohr apparatus at 18 mbar,
furnishing highly pure products as yellowish to colorless liquids.
Characterization was carried out using infrared (IR) spectroscopy, measured in
cm–1. Analytical results were compatible with the planned molecules
and are disclosed below.
3a: Rf 0.56 (AcOEt/Hex 7:3), yield 42,7%, bp 98-100 °C, IR:
2958, 2851 (C-H), 1730 (C=O);
3b: Rf 0.67 (AcOEt/Hex 1:1), yield 81%, bp 110-112°C, IR: 2934
(C-H), 1736 (C=O);
3c: Rf 0.79 (AcOEt/Hex 7:3), yield 68%, bp 124-126°C, IR: 2923
(C-H), 1736 (C=O);
3d: Rf 0.61 (AcOEt/Hex 1:1), yield 62%, bp 132-134°C, IR:
2935, 2795 (C-H), 1748 (C=O);
3e: Rf 0.55 (AcOEt/MeOH 1:1), yield 80%, bp 133-135°C, IR:
2940, 2810 (C-H), 1731 (C=O);
3f: Rf 0.36 (AcOEt), yield 68%, mp 42-44°C, IR:
2980, 2905 (C-H), 1746 (C=O);
3g: Rf 0.61 (AcOEt/Hex 7:3), yield 81%, bp 120-122°C, IR: 2945
(C-H), 1740 (C=O);
3h: Rf 0.65 (AcOEt/Hex 1:1), yield 78%, bp 152-154°C, IR: 2940
(C-H), 1742 (C=O);
3i: Rf 0.70 (AcOEt/Hex 1:1), yield 64%, bp 127-127°C, IR: 2924
(C-H), 1741 (C=O).
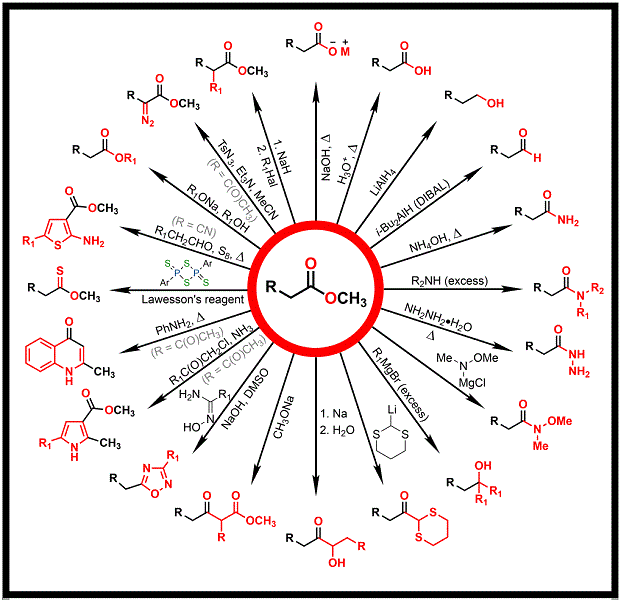
Figure 1. Synthetic applications of esters
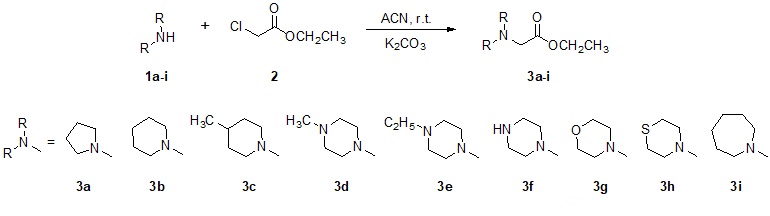
Scheme 1. Synthetic route for 3a-i
Conclusões
Herein a successful methodology for the synthesis of 9 ethyl N- cycloamine acetates was developed. This series of molecules is of synthetic interest since they are building blocks for the synthesis of several other molecules with potential biological activity. The multigram methodology allowed the determination of the boiling points of all products, establishing this characterization parameter for the first time under standardized conditions.
Agradecimentos
The authors are grateful to the Analytical Centre of Fundamental Chemistry Department, Universidade Federal de Pernambuco, for the spectroscopic experiments.
Referências
CHEMINFOGRAPHIC, https://cheminfographic.wordpress.com/2022/04/06/esters/.
ERDAG, D.; MERHAN O., YILDIZ B. Biochemical and Pharmacological Properties of biogenic Amines. In: Biogenic Amines, IntechOpen, 2019.
HAMEED, A.; JAVED, S.; NOREEN, R.; HUMA, T.; IQBAL, S.; UMBREEN, H.; GULZAR, T.; FAROOQ, T. Facile and Green Synthesis of Saturated Cyclic Amines. Molecules, 22, 1691, 2017.
MESHRAM, H.M.; REDDY, B.C.; GOUD, P.R. Triton B–Mediated Mild, Convenient, and
Efficient Method for the Selective Alkylation of Cyclic Secondary Amines and Thiols. Synthetic Communications, 39, 2297-2303, 2009.
SALEH, M.M.; LAUGHTON, C.A.; BRADSHAW, T.D.; MOODY, C.J. Development of a series of bis-triazoles as G-quadruplex ligands. RSC Adv., 7, 47297-47308, 2017.