Autores
Luz, J.R.D. (UNIVERSIDADE DO ESTADO DO AMAPÁ) ; Rabelo, C.W.R. (UNIVERSIDADE DO ESTADO DO AMAPÁ) ; Nascimento, T.E.S. (UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE) ; Barbosa, E.A. (UNIVERSIDADE DE BRASÍLIA) ; Ururahy, M.A.G. (UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE) ; López, J.A. (UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE) ; Almeida, M.G. (UNIVERSIDADE FEDERAL DO RIO GRANDE DO NORTE) ; Silva, G.A. (UNIVERSIDADE DO ESTADO DO AMAPÁ)
Resumo
The anti-inflammatory properties of Licania rigida Benth have been evaluated as
an alternative drug approach to treating several inflammatory processes. In this
study, aqueous and hydroalcoholic extracts of L. rigida leaves were analyzed by
LC-MS/MS, and their anti-inflammatory properties were assessed by an in vivo
inflammation model using LPS as an inducer. The phytochemical profile revealed
gallic and ellagic acids as the main constituent in both extracts. The extracts
displayed the ability to modulate the in vivo inflammatory response by changing
the pro-inflammatory cytokines secretion (TNF-α, IL-1β, and IL-6), also
inhibiting both NO production and leukocyte migration. Overall, results
highlight and identify the ability of L. rigida leaf extracts to modulate the
inflammatory process.
Palavras chaves
Plant extract; phytocomposition; anti-inflammatory
Introdução
The inflammatory process provides a complex series of biochemical cellular
events, tightly controlled, that evolve to eliminate or contain foreign
infectious agents and repair tissue damage. This response is normally beneficial
and necessary for the organism as a self-regulating process to restore
homeostasis in a short time. An inefficient or uncontrolled response of this
system promotes cellular dysfunction, tissue damage, and inadequate repair,
which are characteristic of many inflammatory diseases (CHAN et al, 2022).
Although important for human body, these responses must be efficiently regulated
to prevent the development and worsening of inflammatory diseases. Thereby,
several cellular mediators are secreted to perform essential functions for
achieving homeostasis. White blood cell infiltration is pivotal for the
inflammatory process (PIRLAMARLA et al, 2016).
An inefficient or decompensated response contributes to cellular dysfunction,
tissue damage, and inadequate repair detected in many inflammatory diseases.
Thus, during an exacerbated response, the use of anti-inflammatory drugs is
required as an attempt to hinder deleterious effects on the human body. Hence,
non-steroidal anti-inflammatory drugs (NSAIDs) are applied clinically, although
their prolonged use cause serious side effects, such as iron deficiency anemia,
gastric ulcers, liver, and kidney toxicity, as well as gastrointestinal
bleeding, with a concomitant increase in morbidity and mortality rates. It is
worrying once anti-inflammatory drugs are used indiscriminately worldwide by
individuals of all age groups. Thereby, studies have focused on natural
compounds as an alternative treatment to modulate the inflammatory response,
aiming at the search for molecules with relatively few side effects, especially
for long-term use (PIRLAMARLA et al, 2016; SIREGAR et al, 2021). In this
scenario, medicinal plants are a reservoir of chemical substances, whose
therapeutic properties in the human body to be carefully analyzed. Many of these
plant substances, called active principles, are transformed into drugs suitable
for treating several human diseases (ATANASOV et al, 2021).
In this context, the Brazilian biodiversity stands out worldwide, with
approximately 46.000 cataloged species. One of these biomes is the caatinga,
whose vegetation is poorly researched, requiring studies to elucidate and ensure
the rational and safe use of plant species to which folk medicine attributes
pharmacological properties. Based on this, Licania rigida Benth is a large
evergreen tree species from the Brazilian caatinga, known as oiticica, which
points up due to its popular use in the treatment of inflammatory processes and
diabetes (ALBUQUERQUE et al, 2007). Furthermore, this plant is traditionally
used for its antimicrobial and anticancer properties, which are associated with
oxidative stress (PESSOA et al, 2016; MORAIS et al, 2022). Regarding plants
belonging to the same family as Licania (Chrysobalanaceae), studies have
evaluated biological and pharmacological activities, demonstrating efficient
anti-inflammatory effects (SANTOS et al, 2021).
Despite the role of medicinal plants as a strategy for the treatment and
prevention of diseases due to their pharmacological properties, a constant
concern regarding their use is toxicity, cytotoxicity, genotoxicity and
mutagenicity. It is already proven that many plant species have toxic
constituents, responsible for triggering hepato- and renal toxic effects,
abortion and even poisoning (OLIVEIRA et al, 2020). In this context, studies
show no toxic, cytotoxic, or genotoxic effects in vivo and in vitro using L.
rigida alcoholic and aqueous leaf extracts. Therefore, the use of these extracts
is promising and safe plant from a toxicological point of view (LUZ et al, 2021;
BATISTA et al, 2021).
Based on the above considerations, L. rigida displays promising pharmacological
activities described in the literature. Nonetheless, data regarding this plant
require a deeper analysis of these activities due to its use indiscriminate in
folk medicine and also to the urgent need for alternatives to anti-inflammatory
therapy, considering the undesirable reactions resulting from conventional
treatment with NSAIDs. Therefore, studies on the anti-inflammatory potential of
plant species are relevant to elucidate their phytocomposition and possible
pharmacological application. Hence, this study analyzed the chemical composition
of L. rigida leaf extracts and evaluated their anti-inflammatory effects by
applying an in vivo model of LPS-induced peritonitis as a contribution to the
prospection of new anti-inflammatory molecules with low side effects.
Material e métodos
Collection of plant material and preparation of extracts
L. rigida leaves were collected in Florânia - RN, Brazil in April 2021 under
aproval of SisBio and SisGen. The species was identified at Herbarium of Federal
University of Rio Grande do Norte, Natal - RN, Brazil under registration number
0674/08.
After selection, leaves were cleaned and air-dried at 40°C for 48 h. Then, 300 g
of powdered material were subjected to decoction (100°C/10 min) in water (1:10,
w/v), filtered and lyophilized to obtain aqueous extract (AELR). Respecting the
hydroethanolic extract, 300 g of powdered leaves were macerated with 1.5 L
ethanol: water (50:50, v/v) for four days at room temperature. The extracts were
filtered, rotaevaporated, and lyophilized, denominating HELR.
Phytohemical Analysis by LC–MS/MS
Sample analysis was performed by ultrafast liquid chromatography in a UPLC
Eksigent UltraLC 110-XL liquid chromatograph (AB Sciex, Framingham, MA, USA)
coupled to Kinetex 2.6 µm C18 100 Å column (50 × 2.1 mm) and a 5600+ TripleT
spectrometer (AB Sciex, Framingham, MA, USA). GNPS platform were used for
analysis with the Molecular-Library Search-V2 (version release_14) tool. Data
were filtered by removing peaks with ~17 Da. Then, data were grouped by the MS-
Cluster with tolerances to an original mass of 0.02 Da and an ion of MS/MS
fragments of 0.1 Da to create consensus spectra.
Animals
C57BL/6 male mice (25-30 g) were obtained from the UFRN Vivarium. All
experiments were approved by the UFRN Ethics Committee on Animal Use (Protocol
No. 254.021/2021).
Leukocyte migration into peritoneal cavity
C57BL/6 male mice were divided into four groups (n = 5) as follows: Group 1,
negative control, receiving only PBS solution; Group 2, positive control, and
Groups 3 and 4, treated with 25mg/Kg of AELR and HELR, respectively. Groups 2,
3, and 4 were stimulated intraperitoneally with 2 µg/mL of LPS (E. coli O55:B5
strain) to induce acute inflammation. After 15 min, doses of AELR and HELR (25
mg/kg) were administered intravenously to groups 3 and 4. Four hours later, mice
were anesthetized with xylazine and ketamine (1:1) and euthanized, washing the
abdominal cavity with 2 mL of 0.5% saline solution and 1mM EDTA before
collecting peritoneal fluids. After recovery, total cells were counted in a
hemocytometer while the differential polymorphonuclear leukocyte (PMN) count was
determined in eosin- and hematoxylin-fixed cytospin preparations.
Cytokine Measurement (TNF-α, IL1-β, IL-6)
The collected peritoneal fluid TNF- α, IL1-β, and IL-6 levels from each group
after LPS-induced inflammation were measured using the enzyme-linked
immunosorbent assay (ELISA) kit (eBioscience) following the manufacturer's
instructions. The OD was performed in triplicate at 450nm.
Measurement of Nitric Oxide (NO) Production
The total NO concentration was assessed after the addition of Griess reagent to
100 µL of peritoneal fluid and measuring the absorbance at 545 nm. All readings
were performed in triplicate using a Microplate ELISA Reader (Epoch-Biotek,
Winooski, VT, USA).
Statistical Analysis
Data were expressed as mean ± SEM and analyzed with one-way ANOVA and Tukey’s
post hoc test, using GraphPad Prism version 6.0 Software for Windows (GraphPad
Software, San Diego, CA, USA). p < 0.05 was considered statistically
significant.
Resultado e discussão
Despite triggering undesirable adverse effects such as gastrointestinal
bleeding, NSAIDs are widely used clinically as anti-inflammatory drugs. These
drugs are used to treat intestinal inflammation, including irritable bowel
syndrome (IBS), which corresponds to a group of chronic inflammatory diseases,
such as Crohn's Disease and Ulcerative Colitis, in which NSAIDs can exacerbate
these pathologies. Moreover, the chronic use of this class of drugs is
responsible for the development of these pathologies, and patients often use
several drugs to treat inflammation. Nowadays, patients have an aggravating
factor due to the unavailability of effective drugs with low side effects for
these disease management. Hence, studies have evidenced the effectiveness of
natural and herbal products for Crohn's disease and ulcerative colitis treatment
(MANINUOLA et al, 2018; HUANG et al, 2022).
It is also noteworthy that NSAIDs are responsible for causing hypersensitivity
reactions in patients, which can result in anaphylaxis and death (TRINH et al,
2021). The indiscriminate use of NSAIDs stimulates the search for new
therapeutic methods, and, in this context, medicinal plants represent a
reservoir of chemical compounds with great potential to be explored for the
development and production of new and effective drugs (NUNES et al, 2020).
The bioactivities attributed to phytochemical compounds generate great
scientific interest for further studies due to several therapeutic properties,
including antioxidant and anti-inflammatory effects (SHAZHNI et al, 2018).
Hence, this study analyzed the phytocomposition of L. rigida aqueous and
hydroethanolic leaf extracts by mass spectrometry, also evaluating their anti-
inflammatory in vivo models.
L. rigida aqueous and hydroethanolic leaf extracts were analyzed by LC-MS/MS and
their spectra were submitted to the GNPS database in order to identify the
detected compounds. Despite the high number of MS/MS spectra acquired for each
extract, only spectra matching with cosine ≥0.85 and a mass difference ≤0.005
concerning molecules deposited in the GNPS database were considered for this
analysis. Both extract chemical profiles showed the presence of gallic acid, a
metabolite of pharmacological interest, besides ellagic acid. Additionally,
other constituents were evidenced such as adenosine monophosphate,
phenylalanine, vitamin B6 (pyridoxine), and isovitexin. Furthermore, the
antioxidant ferulic acid, and pheophorbide A, and a lactic acid derivative were
identified in the hydroalcoholic extract.
Extracted ion chromatograms (XICs) obtained for each structure and identified by
UPLC–MS/MS and GNPS analyses showed four main phytocomponents, with a clear
resolution for the L. rigida aqueous extracts (Figure 1A), as well as nine
structures in hydroethanolic extracts (Figure 1B).
Studies concerning the phytochemical characterization of the genus Licania are
scarce in scientific literature. However, literature revealed the presence of
tritepernoids, diterpenoids, steroids, and flavonoids, as the main chemical
compounds in the Chrysobalanacea family (CARNEVALE et al, 2013). Meanwhile,
studies detected significant amounts of phenolic compounds and flavonoids with
flavonol-3-O-glycosylates as main constituent in phytochemical analysis of L.
rigida hydroalcoholic leaf extract and its ethyl acetate fraction (MORAIS et al,
2022). This flavonol is probably isovitexin, identified in the hydroalcoholic
extract of the present study, although further analyzes, such as NRM are
required to confirm this structure. Moreover, other studies have analyzed
different extracts and fractions of L. rigida leaves and seeds, identifying
catechins, chalcones, flavonoids, and tannins in their chemical profiles (SANTOS
et al, 2021). The AELR and HELR phytochemical analysis also identified compound
classes like those described in these studies.
Leukocyte migration in the inflammatory process is of paramount importance since
it is responsible for the induction, maintenance, and regulation of immune
responses (KAMERITSCH et al, 2020). Regarding the leukocyte count, AELR and HELR
extracts significantly decreased the leukocyte expression amount after
treatment, both in total and differential leukocyte counts (Figure 2A and 2B).
Acute inflammation was LPS-induced in the peritoneal cavity of C5,7BL/6 male
mice to determine the L. rigida extract ability to inhibit the inflammatory
cytokine infiltration at the injury site. Thus, LPS-stimulated animals (positive
control) showed an inflammatory cytokine significant increase in the peritoneal
cavity (p<0.05) compared to non-stimulated group (negative control), confirming
the inflammatory process induced by LPS (Figure 2C, 2D and 2E). Regarding
animals with LPS-induced inflammation, their treatment with the aqueous and
hydroethanolic extracts showed a significant decrease in the inflammatory
cytokine infiltration into the peritoneal cavity (p<0.05). Both aqueous and
hydroethanolic extracts inhibited the TNF-α secretion, although HELR displayed a
more satisfactory result with a reduction around 50% (Figure 2C). Respecting the
IL-1β secretion, both extracts reduced this secretion, highlighting the HELR for
its ability to inhibit it in values above 50% (Figure 2D). Both extracts reduced
the IL-6 secretion by about 50% (Figure 2E).
Respecting the NO secretion, a local inflammation was LPS-stimulated in the male
mouse peritoneal cavity. Animals stimulated with LPS (positive control) showed a
significant NO increase in the peritoneal cavity (p<0.05) compared to non-
stimulated animals (negative control), confirming the inflammatory process
development (Figure 2F). However, after AELR and HELR treatments, a reduction in
NO production by around 50% was observed in the peritoneal cavity LPS-stimulated
animals.
The anti-inflammatory effect of L. rigida extracts also displayed a leukocyte
migration decrease and a pro-inflammatory cytokine inhibition in the peritoneal
cavity. A similar result shows the anti-inflammatory activity of L. rigida
hydroethanolic leaf extract in mouse systemic inflammation model (SANTOS et al,
2019). Probably, in both cases, the observed anti-inflammatory effect was due to
the extract polyphenol contents.
Hence, the efficient anti-inflammatory effect evidenced after L. rigida extract
treatments suggests a synergistic effect due to the different compounds
identified in AELR and HELR. Studies have shown that drug combination is a
strategy since synergism offers opportunities to improve the treatment
effectiveness (PEMOVSKA et al, 2018). Regarding plants, this synergy occurs
since extracts are a mixture of secondary metabolites, which can interact with
each other, resulting in a robust control to treat diseases (ZHANG et al, 2019).
Synergistic anti-inflammatory interactions of phytochemicals have been reported
in studies, indicating the combined effects of these phytocompounds or their
synergistic interactions to ameliorate an inflammatory process (YUAN et al,
2017).
Overall, natural products comprise a diversity of compounds, which can interact
with different targets. Furthermore, some components of this phytocomposition
can function as additives or as synergists to exhibit their therapeutic effects
associated with other bioactive co-actives (LUZ et al, 2022). Therefore, natural
products as a complex mixture of molecules have aroused and attracted scientific
interest, considering the potential for synergistic therapeutic effects of their
chemical compositions (ELMAIDOMY et al, 2020).
Experimental data show the L. rigida pharmacological potential, evidenced by the
anti-inflammatory effect of both aqueous and the hydroethanolic extracts,
efficient in reducing leukocyte migration and modulating the inflammatory
cytokine expression. Thereby, chemical and biological results suggest its
potential for prospecting safe molecules and formulations to be applied in the
therapeutic management of inflammatory processes.
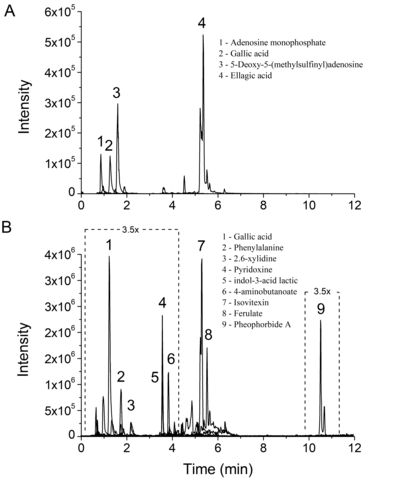
Figure 1. LC–MS/MS fingerprint of L. rigida extracts: (A) L. rigida aqueous leaf extract; (B) L. rigida hydroethanolic leaf extract. 3.5× denote the magnification applied in the chromatogram dotted areas.
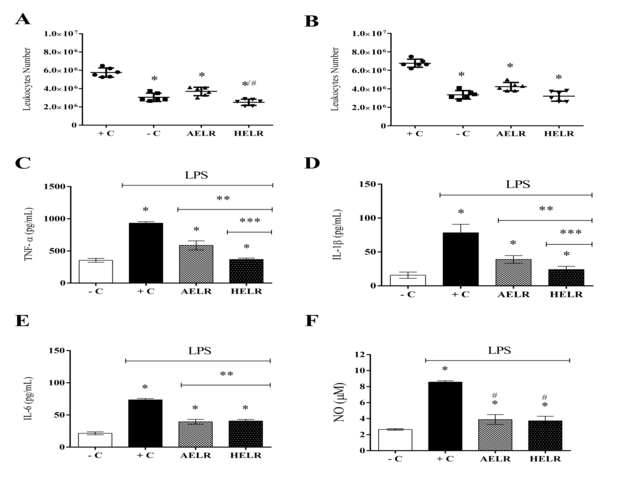
Figure 2. Total Leukocyte (A) and Differential leukocyte (B). L. rigida aqueous extract (AELR); L. rigida hydroethanolic extract (HELR), - C (negative control – animals not induced with LPS) and + C (positive control – animals induced with LPS a
Conclusões
The present study investigated the phytochemical analysis by LC(MS/MS) of L.
rigida aqueous and hydroethanolic leaf extracts, which showed a rich composition
in phenolic compounds as well as flavonoids. Furthermore, these extracts were able
to promote a significant anti-inflammatory effect in an in vivo model of LPS-
induced peritonitis. AELR and HELR displayed a marked reduction in leukocyte
migration to the mouse peritoneal cavity, besides a reduction in the expression of
inflammatory cytokines. L. rigida extracts also inhibited NO production. The
results suggest an action associated with the inhibition of cytokine production as
well as the extract phytocomposition that may be responsible for the evidenced
anti-inflammatory activity. Although further studies are required, data provide
promising evidence supporting AELR and HELR as alternatives in prospecting
potential ant-inflammatory agents.
Agradecimentos
The authors would like to thank the CNPq for providing post-graduation fellowship
(Process No. 169246/2018-3) and Federal University of Rio Grande do Norte (Grant
No. 397/2020).
Referências
ALBUQUERQUE, U.P.; MEDEIROS, P.M.; ALMEIDA, A.L.S.; MONTEIRO, J.M.; LINS NETO, E.M.F.; MELO, J.G.; SANTOS, J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol, 114, 325-354, 2007.
ATANASOV, A.G.; ZOTCHEV, S.B.; DIRSCH, V.M. The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov, 20, 200-216, 2021.
BATISTA, D.; LUZ, J.R.D.; NASCIMENTO, T.E.S.; SENES-LOPES, T.F.; GALDINO, O.A; SILVA, S.V.; FERREIRA, M.P.; AZEVEDO, M.A.S.; BRANDÃO-NETO, J.; ARAUJO-SILVA, G. Licania rigida leaf extract: Protective effect on oxidative stress, associated with cytotoxic, mutagenic and preclinical aspects. J. Toxicol. Environ. Health Part A, 20, 276-290, 2021.
CARNEVALE, N.F.; PILON, A.C.; SILVA B.V. Chrysobalanaceae: secondary metabolites, ethnopharmacology and pharmacological potential. Phytochem. Rev, 12, 121-146, 2013.
CHAN, J.T.H.; KADRI, S.; KÖLLNER, B.; REBL, A.; KORYTÁŘ, T. RNA-seq of single fish cells - Seeking out the leukocytes mediating immunity in teleost fishes. Front. Immunol, 13, 1664-3224, 2022.
ELMAIDOMY, A.H.; ALHADRAMI, H.A.; AMIN, E.; ALY, H.F.; OTHMAN, A.M.; RATEB, M.E.; HETTA, M.H.; ABDELMOHSEN, U.R.; HASSAN, H.M. Anti-inflammatory and antioxidant activities of terpene- and polyphenol-rich Premna odorata leaves on alcohol-inflamed female Wistar albino rat liver. Molecules, 25(14), 3116, 2020.
HUANG, P.; HONG, J.; MI, J.; SUN, B.; ZHANG, J.; LI, C.; YANG, W. Polyphenols extracted from Enteromorpha clathrata alleviates inflammation in lipopolysaccharide-induced RAW 264.7 cells by inhibiting the MAPKs/NF-κB signaling pathways. J. Ethnopharmacol, 286, 114897, 2022.
KAMERITSCH, P.; RRNKAWITZ, J. Principles of leukocyte migration strategies. Trends Cell Biol, 30, 818-832, 2020.
LUZ, J.R.D.; NASCIMENTO, T.E.S.; ARAUJO-SILVA, G.; REZENDE, A.A.; BRANDÃO-NETO, J.; URURAHY, M.A.G.; LUCHESSI, A.D.; LÓPEZ, J.A.; ROCHA, H.A.O.; ALMEIDA, M.G. Licania rigida Benth leaf extracts: Assessment of toxicity and potential anticoagulant effect. S. Afr. J. Bot, 139, 217-225, 2021.
LUZ, J.R.D.D.; BARBOSA, E.A.; NASCIMENTO, T.E.S.D.; REZENDE, A.A.D.; URURAHY, M.A.G.; BRITO, A.D.S.; ARAUJO-SILVA, G.; LÓPEZ, J.A.; ALMEIDA, M.D.G. Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages. Molecules, 27, 1084, 2022.
MONINUOLA, O.O.; MILLIGAN, W.; LOCHHEAD, P.; KHALILI, H. Systematic review with meta-analysis: association between acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) and risk of Crohn’s disease and ulcerative colitis exacerbation. Aliment. Pharmacol. Ther, 47, 1428-1439, 2018.
MORAIS, L.V.F.; LUZ, J.R.D.; NASCIMENTO, T.E.S.; AZEVEDO, M.A.S.; ROCHA, W.P.S.; ARAUJO-SILVA, G.; URURAHY, M.A.G.; CHAVES, G.M,; LÓPEZ, J.A.; SANTOS, E.C.G.; ALMEIDA, M.G. Phenolic composition, toxicity potential, and antimicrobial activity of Licania rigida Benth (Chrysobalanaceae) leaf extracts. J. Med. Food, 25, 97-109, 2022.
NUNES, C.R.; ARANTES, M.B.; PEREIRA, S.M.P.; CRUZ, L.L.; PASSOS, M.S.; MORAES, L.P.; VIEIRA, I.J.C.; OLIVEIRA, D.B. Plants as sources of anti-inflammatory agents. Molecules, 25, 3726, 2020.
OLIVEIRA, M.C.B.; CRUZ, C.K.S.; ROCHA, G.M.M.; BRITO, M.G.A.; OLIVEIRA, G.A.L. Toxicity and antibacterial activity of medicinal plants used in the treatment of respiratory diseases: an integrative review. Res. Soc. Dev, 9, e244997169, 2020.
PEMOVSKA, T.; JOHANNES, W.B.; SUPERTI-FURGA, G. Recent advances in combinatorial drug screening and synergy scoring. Curr. Opin. Pharmacol, 42, 102-110, 2018.
PESSOA, I.P.; LOPES NETO, J.J.; ALMEIDA, T.S.; FARIAS, D.F.; VIEIRA, L.R.; MEDEIROS, J.L.; BOLIGON, A.A.; PEIJNENBURG, A.; CASTELAR, I.; CARVALHO, A.F.U. Polyphenol composition, antioxidant activity and cytotoxicity of seeds from two underexploited wild Licania species: L. rigida and L. tomentosa. Molecules, 21(12), 1755, 2016.
PIRLAMARLA, P.; BOND, R.M. FDA labeling of NSAIDs: Review of nonsteroidal anti-inflammatory drugs in cardiovascular disease. Trends Cardiovasc. Med, 26, 675-680, 2016.
SANTOS, E.S.; OLIVEIRA, C.D.M.; MENEZES, I.R.A.; NASCIMENTO, E.P.; CORREIA, D.B.; ALENCAR, C.D.C.; SOUSA, M.F.; LIMA, C.N.F.; MONTEIRO, A.B.; SOUZA, C.P.E.; DELMONDES, G.A. Anti-inflammatory activity of herb products from Licania rigida Benth. Complement. Ther. Med, 45, 254-261, 2019.
SANTOS, E.S.; OLIVEIRA-TINTINO, C.D.M.; CORREIA, D.B.; ALENCAR, C.D.C.; SOUSA, M.F.; LIMA, C.N.F.; MACHADO, S.T.S.; GOMES, A.D.S.; GARCIA, F.A.O.; MENEZES, IRA. Topical anti-inflammatory effect of hydroalcoholic extract of leaves of Licania rigida Benth. in mice. Phytomedicine Plus, 1, 100110, 2021.
SHAZHNI, J.R.A.; RENU, A.; VIJAYARAGHAVAN, P. Insights of antidiabetic, anti-inflammatory and hepatoprotective properties of antimicrobial secondary metabolites of corm extract from Caladium x hortulanum. Saudi J. Biol. Sci, 25, 1755-1761, 2018.
SIREGAR, A.S.; WERDHANI, R.A.; ASCOBAT, P.; NAFRIALDI, N.; SYAM, A.F.; HIDAYAT, R.; WANGGE, G. Development of module for the prevention of nonsteroidal anti-inflammatory drugs-associated gastrointestinal adverse reactions in the elderly at a primary health center. Int. J. Risk Saf. Med, 32, 61-73, 2021.
TRINH, H.K.T.; PHAM, L.D.; LE, K.M.; PARK, H.S. Pharmacogenomics of hypersensitivity to non-steroidal anti-inflammatory drugs. Front. Genet, 12, 647257, 2021.
VENANCIO, V.P., CIPRIANO, P.A., KIM, H., ANTUNES, L.M.G., TALCOTT, S.T.; MERTENS-TALCOTT, S.U. Cocoplum (Chrysobalanus icaco L.) anthocyanins exert anti- inflammatory activity in human colon cancer and non- malignant colon cells. Food Funct, 8, 307-331, 2017.
YUAN, H.; MA, Q.; CUI, H.; LIU, G.; ZHAO, X.; LI, W.; PIAO, G. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Molecules, 22(7), 1135, 2017.
ZHANG, L.; VIRGOUS, C.; SI, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem, 69, 19-30, 2019.
















