Autores
Firmino, P.P. (IFSC/USP) ; Santiago, P.H.O. (IFSC/USP) ; Ellena, J. (IFSC/USP)
Resumo
This work addresses the obtaining, crystallographic, thermal characterization, and
relative solubility of Hydralazine. Hydralazine is sold in the form of hydralazine
hydrochloride and is used as an antihypertensive. The free form of hydralazine is
not yet reported in the Cambridge Crystallographic Data Centre-Cambridge
Structural Database (CSD 2022.2), which motivated the study. The results of the
single crystal X-ray were compared with the results obtained by the powder X-ray
indicating a high purity of the result of the HCl extraction which agree with the
differential calorimetry analysis. The relative solubility tests in purified water
at room temperature showed that the saturated concentration of hydralazine is 2.04
mg/ml, being less soluble than hydralazine hydrochloride.
Palavras chaves
Crystallography; Relative solubility; Thermal characterization
Introdução
Hydralazine is an antihypertensive drug with vasodilator properties that has
been used in the treatment of arterial hypertension since the 1950s, being
usually the first choice for the acute treatment of severe arterial hypertension
in pregnancy. This drug is also used in cases of treatment of eclampsia and
heart failure and can be used in the treatment of some types of cancer to
support chemotherapy due to its demethylating effect on various suppressor
genes(VANITHA; VARMA; RAMESH, 2013). Recent studies demonstrate that hydralazine
has antioxidant and antiapoptotic potential, also conferring acute
cardioprotection for patients by inhibiting mitochondrial fission induced by
acute myocardial ischemia/reperfusion injury, which can be repurposed in
cardioprotective therapy to improve post-infarction outcome (KALKHORAN et al.,
2022; MCCOMB; CHAO; NG, 2015).
Normally the hydralazine is commercially available as hydralazine hydrochloride
(Hyd·HCl) and is sold in Brazil as Apresoline with 25 mg or 50 mg, the physical
characteristic of powder is white or almost white placement; it has a half-life
of 2 to 4 hours with an oral bioavailability of 26-50% (VANITHA; VARMA; RAMESH,
2013). The primary mechanism of action of Hydralazine is based on its
characteristic of being a direct vasodilator, relaxing the smooth muscle cell of
the vascular wall at the arteriolar level, thus causing a hypotensive effect,
with the ability to bind to plasma proteins (mainly albumin) between 88 and 90%
(EBEIGBE; ALOAMAKA, 1985; JACOBS, 1984).The crystal structure of Hyd·HCl was
previously reported at the Cambridge Crystallographic Data Centre-Cambridge
Structural Database (CSD 2022.2)(BRUNO et al., 2002), but the free hydralazine
had not yet its crystal structure elucidated. Knowing this, we decided to obtain
the free hydralazine form to elucidate the crystal structure
and evaluate the difference in the physicochemical properties of the free Hyd
and the Hyd·HCl.
The effectiveness of a drug is mainly associated with its properties in the
solid-state (NARALA et al., 2022; XUAN et al., 2021).The knowledge of molecular
conformation and supramolecular arrangement is a useful acknowledgment for
understanding the chemical, physicochemical, and biological properties for a
compound, leading from the structural characterization to the application of
crystal engineering that plays an important role in many pharmaceutical
industries, so that new solid-state forms of active drug ingredients (APIs) can
be designed in order to modulate properties such as solubility, bioavailability,
flow, compressibility, thermal stability, crystallinity and hygroscopicity,
among many others(SOUZA et al., 2019).
Solubility is the property of a solute to dissolve in a solvent to form a
solution, depending directly of the solvent used, temperature and pressure. The
degree of solubility of a substance in a specific solvent is measured as the
saturation concentration where the addition of more solute does not increase its
concentration in the solution. The solubility is also a property of a drug that
affects the bioavailability of the drug product, being important because of the
most convenient and commonly route of drug delivery is the oral ingestion, being
an easy way of administration the drug (AGUIAR et al., 2020).
In particular, the aqueous solubility of a drug is a prerequisite for
absorption, which is an important barrier to the effectiveness of a drug when
its water solubility is low(BISCAIA et al., 2021; ROLIM-NETO et al., 2015).
Knowing this, we bring the process to obtain the free form of hydralazine, the
solid-state characterization by single crystal X-ray diffraction (SCDRX) and X-
ray powder diffraction (DRXP), the thermal behavior description using
Differential scanning Calorimetry (DSC) and Thermogravimetric analysis (TGA),
and relative solubility in water measured with UV-Vis spectroscopy. This
acknowledgment is helpful in the development of novel studies for the drug. The
solid-state description of Hyd enables the use of that drug in different field,
such as crystal engineering, where new solid forms with better physicochemical
properties can be developed, aiming the obtaining of new materials with better
bioavailability, implying a reduction in the necessary amount of API to achieve
the same desired effect.
Material e métodos
The free hydralazine was obtained using an acid-base process. NaOH was added to
an aqueous solution of Hyd·HCl in equimolar proportion. The aqueous solution was
mixed with dichloromethane and stirred for some minutes. The organic phase was
separated from the aqueous phase and kept under ambient conditions until the
crystallization of the free Hyd with the slow evaporation of the solvent.
Hydralazine was obtained with a yield of 72%.The X-ray data collection was
accomplished on a XtaLAB Synergy-S Dualflex diffractometer equipped with a
Hypix-6000HE detector,using a Cu Kα radiation (λ = 1.54184 Å), with the crystal
kept at 100 K for the data collection. After the structural elucidation, the
next step was to verify the purity and the conversion of Hyd·HCl into the free
Hyd.For this the macerated sample was analyzed by X-ray powder diffraction
(XRPD) using a Rigaku diffractometer ultima lV with 2θ in the range of 5° to 50°
, with a step of 0.02/second , speed of 50°/min, voltage of 40 kv e 20 mA, using
sealed Cu tube(DINIZ et al., 2020).Analyzes of the reflections collected from
the X-ray diffraction by monocrystal were made, which were solved using the
Olex2 software, the structure was solved with the SHELXT structure solution
program using Intrinsic Phasing and refined with the SHELXL refinement package
using Least Squares minimization(DOLOMANOV et al., 2009a, 2009b; MÜLLER, 2006;
SHELDRICK, 2015).To verify the thermal stability of hydralazine, Differential
Scanning Calorimetry (DSC) measurements and Thermogravimetric Analysis (TGA)
were performed. The TGA was performed in a Shimadzu TGA-50 equipment.
Approximately 3.0 mg ± 0.001 mg were placed in a ceramic container(alumina) and
heated from 25 to 400 ºC at rate of 10 oC/min under an atmosphere of
N2 (50 mL.min-1). To DSC analyses in turn, the Shimadzu
DSC-60 calorimeter was used, and 1.5 ± 0.02 mg of the sample were heated at a
rate of 10 ºC/m in a sealed aluminum pan. The N2 flow was also 50
mL.min-1. The results obtained for both techniques were processed in
the Shimadzu TA-60 software (version 2.20).To obtain solution parameters to
guide a stability sequence, relative tests were performed by the flask
saturation method, which consists of promoting the supersaturation of a solution
in thermodynamic equilibrium. The experiment was carried out by applying the
flask saturation method at room temperature, after magnetic stirring for 48
hours using the Shimadzu UV-1800 spectrometer, the known calibration curves were
constructed with five points, each of them measured in triplicate, using the
values of the highest absorbance peak in λ=210 nm.
Resultado e discussão
The free Hydralazine was obtained with na acid-base process, where an aqueous
solution of Hyd·HCl was treated with a NaOH solution. An amount of
dichloromethane was added to the aqueous solution aiming the extraction of the
hydralazine from the aqueous phase to the organic phase. Yellow crystals were
obtained with the slow evaporation of the solution.Hydralazine crystallized in
the orthorhombic crystal system, with space group P 212121 and the
following cell parameters: a = 6.73780(10 (9) Å; b = 9.6979(2) Å; c = 11.0423(2)
Å; α = γ = 90.0°; β = 90°; γ= 90°, V = 721.53(2) ų, with four (Z) units in the
unit cell and the R-factor of 4.1%, Figure 1(a) illustrate the ORTEP diagram of
the asymmetric unit showing 50% probability of ellipsoids .Free hydralazine has
a planar conformation being strongly stabilized by NH···N hydrogen bonds, which
constitute an asymmetric heterosynton (CH···N, NH···N), which leads to strand
formation along the c axis and an NH···N interaction connecting the strands
along the a axis allowing the organization of the crystal lattice Figure 1(b).
The X-ray powder diffraction pattern of hydralazine was obtained and compared
with that calculated from the CIF (Crystallographic Information File) file
obtained with SCXRD analysis, as showed in Figure 1(c), in order to determine
the purity of the compound and to verify if the reaction occurred completely.
The comparison between the X-ray diffraction patterns of calculated and
experimental powder revealed that both the diffractograms show the same peaks
and are too similar, indicating that just one phase of hydralazine was obtained
with high purity. Hydralazine was evaluated by the following thermal
characterization techniques: DSC and TG Figure 2(a). These analyzes allow to
quantify the energy absorbed or released by the sample, allow measuring the loss
of mass as a function of temperature, being important in determining the thermal
stability of the samples, being used in this work for the differentiation and
identification of crystalline modifications of drugs.The DSC curve for
hydralazine presents just one endothermic signal correspondent to the melting
point with onset at 176.8(2) °C, peak at 179.7(2) °C and endset at 182.5(2) °C,
which indicates a high purity for the compound. The TG curve shows the drug
degradation in three steps starting at 182.4°C(2), mass loss at 215.6(2) °C and
360.4(2) °C ending at approximately 450.8(2) °C. The value of the endset
presented in the DSC, with the beginning of the degradation of the hydralazine
presented in the TG show that the drug starts the degradation process soon after
melting.Equilibrium relative solubility studies were performed to compare the
aqueous solubility of the free hydralazine and the Hyd·HCl. Solubility is
intrinsically related to the absorption process and, consequently, to the
therapeutic efficacy of the drug. Equilibrium solubility is related to the
maximum amount of the drug dissolved in a specific solvent, temperature and pH.
To obtain the concentrations of the drugs, several tests were carried out, for
an estimation via the least squares method, being evaluated the variables
through the coefficient of determination (R²), for this, a calibration curve was
made with five points with different and known concentrations, each of them
measured in triplicate, and an equation of te curve was obtained to verify the
solubility of the compounds.The assays were performed using Milli-Q water, and
the sample with saturated and unknown concentration was measured after 48 hours
in magnetic agitation by the shake flask method, in which excess drug was added
in order to reach saturation. The data used to construct the calibration curve
for free Hydralazine, which can be seen in figure 2(b) are(Concentrations
(mg/ml)= 0.002; 0.004; 0.006;0.008; 0,01 and the Average Absorbance
(nm)=0.444;0.925;1.295;1.685;2.413)and the Average absorbance to unknown
concentration is 0.901 nm. The equation of the line obtained was y = 234.91x -
0.0566 with R² = 0.98, the y is equivalent to the average absorbance, while the
x is equivalent to the concentration in mg/ml, then making the relationship and
substitutions the x found which is the concentration was equal to x=0.00407522.
Taking into account that the saturated sample was diluted for the measurement,
the aqueous solubility at room temperature founded for the free hydralazine was
2.04 mg.ml-1, which is lower than the value of Hyd·HCl, it is
described in the literature as 8.00 mg/ml. ml in water and 6.8 mg/ml in
phosphate buffer with a pH of 6.8 (MUTHUKUMAR; GANAPATHY, 2018; SWAMY et al.,
2017).
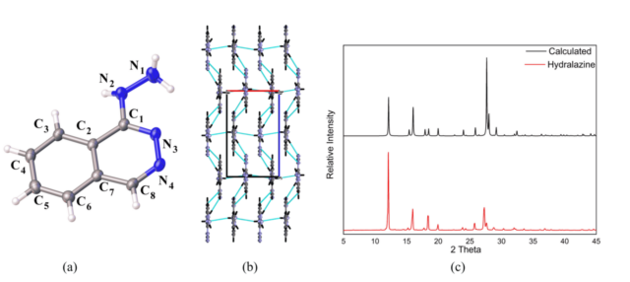
(a) The ortep diagram with 50% probability. (b) Hydrogen bonds stabilizing the hydralazine structure. (c) DRXP comparation results.
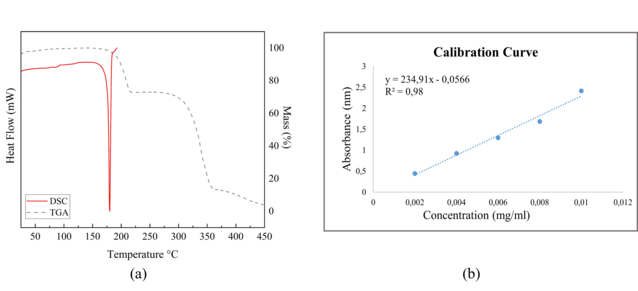
(a) The thermal DSC and TGA curves. (b) The equation of the line though the calibration curve.
Conclusões
Hydralazine is an antihypertensive agent with vasodilator properties, which has
been used since 1950, and is almost used during pregnancy to treat severe
arterial hypertension. Free Hydralazine was obtained from from the Hyd·HCl via
acid-base reaction, and was characterized using SCXRD, XRPD, DSC, TGA and UV-Vis
techniques. The crystallographic data indicate strong NH···N, CH···N and NH···N
interactions, which stabilize the crystalline packing.
The results of the single
crystal X-ray diffraction were compared with the results obtained by the powder
X-ray diffraction, where both of them showed agreement between the peaks
evaluating the experimental and calculated, indicating the high purity of the
result of the HCl extraction. The results of thermal analysis demonstrate that
hydralazine has a single melting peak at 179.7(2) °C as expected for a substance,
starting its degradation process at 182.4(2) °C. The relative solubility tests in
water at room temperature showed that the aqueous solubility of hydralazine is
2.04 mg/ml, being less soluble than Hyd·HCl.
Agradecimentos
The autor thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico
(CNPq 160856/2021-3), and the postgraduate program of Biomolecular Physics of the
Instituto de Física de São Carlos- Universidade de São Paulo-IFSC/USP
Referências
AGUIAR, Antônio S. N.; QUEIROZ, Jaqueline E.; FIRMINO, Pollyana P.; VAZ, Wesley F.; CAMARGO, Ademir J.; DE AQUINO, Gilberto L. B.; NAPOLITANO, Hamilton B.; OLIVEIRA, Solemar S. Synthesis, characterization, and computational study of a new heteroaryl chalcone. Journal of Molecular Modeling, [S. l.], v. 26, n. 9, p. 243, 2020. DOI: 10.1007/s00894-020-04506-1. Disponível em: http://link.springer.com/10.1007/s00894-020-04506-1.
BISCAIA, Isabela Fanelli Barreto; OLIVEIRA, Paulo Renato; GOMES, Samantha Nascimento; BERNARDI, Larissa Sakis. Obtaining cocrystals by reaction crystallization method: Pharmaceutical applications. Pharmaceutics, [S. l.], v. 13, n. 6, 2021. DOI: 10.3390/PHARMACEUTICS13060898. Acesso em: 22 fev. 2022.
BRUNO, Ian J.; COLE, Jason C.; EDGINGTON, Paul R.; KESSLER, Magnus; MACRAE, Clare F.; MCCABE, Patrick; PEARSON, Jonathan; TAYLOR, Robin. New software for searching the Cambridge Structural Database and visualizing crystal structures. urn:issn:0108-7681, [S. l.], v. 58, n. 3, p. 389–397, 2002. DOI: 10.1107/S0108768102003324. Disponível em: http://scripts.iucr.org/cgi-bin/paper?an0609. Acesso em: 21 ago. 2022.
DINIZ, Luan F. et al. Enhancing the solubility and permeability of the diuretic drug furosemide via multicomponent crystal forms. International Journal of Pharmaceutics, [S. l.], v. 587, p. 119694, 2020. DOI: 10.1016/j.ijpharm.2020.119694.
DOLOMANOV, Oleg V.; BOURHIS, Luc J.; GILDEA, Richard J.; HOWARD, Judith A. K.; PUSCHMANN, Horst. OLEX2: A complete structure solution, refinement and analysis program. Journal of Applied Crystallography, [S. l.], v. 42, n. 2, p. 339–341, 2009. a. DOI: 10.1107/S0021889808042726. Acesso em: 25 jun. 2020.
DOLOMANOV, Oleg V.; BOURHIS, Luc J.; GILDEA, Richard J.; HOWARD, Judith A. K.; PUSCHMANN, Horst. OLEX2: A complete structure solution, refinement and analysis program. Journal of Applied Crystallography, [S. l.], v. 42, n. 2, p. 339–341, 2009. b. DOI: 10.1107/S0021889808042726. Acesso em: 12 fev. 2020.
EBEIGBE, A. B.; ALOAMAKA, C. P. Mechanism of hydralazine-induced relaxation of arterial smooth muscle. Cardiovascular Research, [S. l.], v. 19, n. 7, p. 400–405, 1985. DOI: 10.1093/cvr/19.7.400.
JACOBS, M. Mechanism of action of hydralazine on vascular smooth muscle. Biochemical Pharmacology, [S. l.], v. 33, n. 18, p. 2915–2919, 1984. DOI: 10.1016/0006-2952(84)90216-8.
KALKHORAN, Siavash Beikoghli et al. Hydralazine protects the heart against acute ischaemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission. Cardiovascular Research, [S. l.], v. 118, n. 1, p. 282–294, 2022. DOI: 10.1093/cvr/cvaa343. Disponível em: https://academic.oup.com/cardiovascres/article/118/1/282/6059217.
MCCOMB, Meghan N.; CHAO, James Y.; NG, Tien M. H. Direct Vasodilators and Sympatholytic Agents. Journal of Cardiovascular Pharmacology and Therapeutics, [S. l.], v. 21, n. 1, p. 3–19, 2015. DOI: 10.1177/1074248415587969. Disponível em: http://europepmc.org/article/MED/26033778. Acesso em: 21 ago. 2022.
MÜLLER, Peter. Crystal Structure Refinement A Crystallographer’s Guide to SHELXL. Cambridge, USA: Oxford University Press, 2006.
MUTHUKUMAR, S.; GANAPATHY, R. Sundara. FORMULATION AND EVALUATION OF HYDRALAZINE HYDROCHLORIDE BUCCAL FILMS BY SOLVENT CASTING METHOD USING DIFFERENT POLYMERS FOR THE MANAGEMENT OF HYPERTENSION. INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH, [S. l.], v. 9, n. 8, p. 3328–3333, 2018.
NARALA, Sagar; NYAVANANDI, Dinesh; ALZAHRANI, Abdullah; BANDARI, Suresh; ZHANG, Feng; REPKA, Michael A. Creation of Hydrochlorothiazide Pharmaceutical Cocrystals Via Hot-Melt Extrusion for Enhanced Solubility and Permeability. AAPS PharmSciTech, [S. l.], v. 23, n. 1, p. 1–11, 2022. DOI: 10.1208/S12249-021-02202-8/FIGURES/9. Disponível em: https://link.springer.com/article/10.1208/s12249-021-02202-8. Acesso em: 22 fev. 2022.
ROLIM-NETO, Pedro José et al. Estratégias utilizadas para o incremento da solubilidade do fármaco antiretroviral classe II: Efavirenz. Revista de Ciências Farmacêuticas Básica e Aplicada Journal of Basic and Applied Pharmaceutical Sciences Rev Ciênc Farm Básica Apl, [S. l.], v. 36, n. 2, p. 239–249, 2015.
SHELDRICK, George M. Crystal structure refinement with SHELXL. Acta Crystallographica Section C: Structural Chemistry, [S. l.], v. 71, n. Md, p. 3–8, 2015. DOI: 10.1107/S2053229614024218.
SOUZA, Matheus S.; DINIZ, Luan F.; ALVAREZ, Natalia; DA SILVA, Cecília C. P.; ELLENA, Javier. Supramolecular synthesis and characterization of crystalline solids obtained from the reaction of 5-fluorocytosine with nitro compounds. New Journal of Chemistry, [S. l.], v. 43, n. 40, p. 15924–15934, 2019. DOI: 10.1039/C9NJ03329G.
SWAMY, Kumara; KUMARA SWAMY, Samanthula; NAGARJUN REDDY, Lokireddy; AGAIAH GOUD, Bairi. Development and in vitro evaluation of bioadhesive buccal tablets of Hydralazine hydrochloride. [s.l: s.n.]. Disponível em: https://www.researchgate.net/publication/320299894.
VANITHA, Kondi; VARMA, Mohan; RAMESH, Alluri. Floating tablets of hydralazine hydrochloride: optimization and evaluation. Brazilian Journal of Pharmaceutical Sciences, [S. l.], v. 49, n. 4, p. 811–819, 2013. DOI: 10.1590/S1984-82502013000400021.
XUAN, Bianfei; CHEN, Yu Chee Sonia; WONG, Kong Ching; CHEN, Ruipeng; LO, Po Sang; LAKERVELD, Richard; TONG, Henry Hoi Yee; CHOW, Shing Fung. Impact of cocrystal solution-state stability on cocrystal dissociation and polymorphic drug recrystallization during dissolution. International Journal of Pharmaceutics, [S. l.], v. 610, 2021. DOI: 10.1016/J.IJPHARM.2021.121239. Acesso em: 22 fev. 2022.
















