Autores
Pereira, F.S. (UNIVERSIDADE FEDERAL DO MARANHÃO) ; Lima, S.L.S. (PONTIFÍCIA UNIVERSIDADE CATÓLICA DO RIO DE JANEIR) ; Tanaka, A.A. (UNIVERSIDADE FEDERAL DO MARANHÃO) ; Garcia, M.A.S. (UNIVERSIDADE FEDERAL DO MARANHÃO) ; Silva, A.G.M. (PONTIFÍCIA UNIVERSIDADE CATÓLICA DO RIO DE JANEIR)
Resumo
The present investigation shows that high catalytic activity can be reached
towards the ORR by employing 1.8 ± 0.7 nm Ir nanoparticles (NPs) deposited onto
MnO2 nanowires surface under low metal loadings (1.2 wt.%). Interestingly, we
observed that the MnO2-Ir nanohybrid presented high catalytic activity for the ORR
close to commercial Pt/C (20.0 wt.% of Pt), indicating that it could obtain
efficient performance using a simple synthetic procedure.
Palavras chaves
Manganese dioxide; iridium; ORR
Introdução
In general, ORR mainly occurs through two pathways, one involving the transfer
of two electrons (2e− ORR), producing H2O2, and the other consisting of the
transfer of four electrons (4e− ORR), yielding H2O as the product [1].
Therefore, developing electrocatalysts to undergo this pathway is critical for
the optimal electrochemical performance of fuel cells. However, the ORR limits
the performance of many electrochemical devices due to its sluggish kinetics,
which results in considerable overpotentials, causing a significant loss in
energy efficiency; thus, the design of transition metal-based nanomaterials is
pervasive [2, 3]. Manganese dioxide (MnO2) is a promising candidate as an
electrocatalyst for ORR because, in addition to the low cost associated with the
abundance in the form of natural ores, it has environmental compatibility, low
toxicity, and variable oxidation states [4]. However, its major limitation is
the low electronic conductivity, which is unfavorable for rapid electron
transfer during the electrochemical process [5]. A strategy to solve the MnO2
low electronic conductivity is to deposit small amounts (<2.0 wt.%) of some
transition metals. Among them, iridium (Ir) is a promising candidate due to its
higher availability than Pt, lower costs, and suitable activities for the ORR
[6].
Material e métodos
Synthesis of MnO2 Nanowires
In a typical procedure, MnSO4·H2O and KMnO4 were dissolved in deionized water.
This solution was transferred to a Teflon-lined stainless steel autoclave that
was heated and stirred and then allowed to cool down to room temperature. The
nanowires were washed with ethanol and water several times by centrifugation and
removal of the supernatant and finally dried in air.
Synthesis of MnO2 Nanowires Decorated with Ir NPs (MnO2–Ir NPs)
MnO2 nanowires and polyvinylpyrrolidone (PVP) were added to EG. The obtained
suspension was transferred to a round-bottom flask and kept under vigorous
stirring. Then, NaBH4 and IrCl3− solutions were sequentially added to the
reaction flask. This mixture was kept under vigorous stirring to produce MnO2–
IrNPs and washed with ethanol and water by rounds of centrifugation and removal
of the supernatant. After washing, the MnO2–Ir NPs were suspended in water.
Electrochemical Studies
Electrochemical experiments for ORR were performed in a conventional three-
electrode cell using a PGSTAT 302 N (Autolab) potentiostat/galvanostat model
controlled by Nova 2.0 soft-ware and the electrode rotation rate by a Pine ASR
rotator. A potassium hydroxide (KOH) solution was used as the electrolyte,
modified glassy carbon as the working electrode, a platinum wire as the counter
electrode, and saturated AgCl/KCl as the reference electrode. A total of 20 μL
of paint (mixed solution containing 5 mg of the material of interest, 1 mg of
methanol with 0.1 mL of Nafion® 5.0% by weight, and 1.4 mL of deionized water,
dispersed by ultrasound for 10 min) was used to modify the surface of the glassy
carbon electrode.
Resultado e discussão
Figure 1 shows SEM and TEM images of the ultrasmall Ir NPs deposited on MnO2
nanowires. A uniform distribution of monodisperse ultrasmall NPs with a narrow
size distribution all over the surface of the nanowire with particles size of
1.8 ± 0.7 nm. High-angle annular dark field images and scanning transmission
electron microscopy analyses were performed to investigate further the structure
and Mn, O, and Ir elemental distributions in the MnO2 decorated with Ir NPs.
Bright-field STEM (Figure 1C) and HAADF-STEM (Figure 1D) images illustrate the
uniform distribution of ultrasmall Ir NPs at the MnO2 nanowires support. No
significant agglomeration was detected. In addition, STEM-EDS elemental mapping
(Figure 1E) confirmed the uniform deposition of ultrasmall Ir NPs of ultrasmall
Ir NPs over the outer surface of the MnO2 nanowires. No morphological changes in
the nanowire shape could be detected after Ir NPs deposition.
When MnO2 nanowires and MnO2-Ir electrocatalyst were compared with the
commercial Pt/C electrocatalyst, one can notice that the MnO2-Ir nanowires
presented a lower onset potential and higher limiting current, showing its
incredible efficiency (Figure 2). We also compared the MnO2-Ir nanowires with
the 20.0 wt.% Pt/C which presented current and potential density close to the Pt
counterpart. The results were presented in current, without considering the mass
of precious metal. However, such a commercial electrocatalyst offers 20 times
more metal loading than the MnO2-Ir electrocatalyst produced in the present
work, showing the effectiveness of our material. The polarization curve of
material is analogous to the Pt/C counterpart, suggesting that the
electrocatalyst can catalyze a 4e− ORR process for reagent molecule.
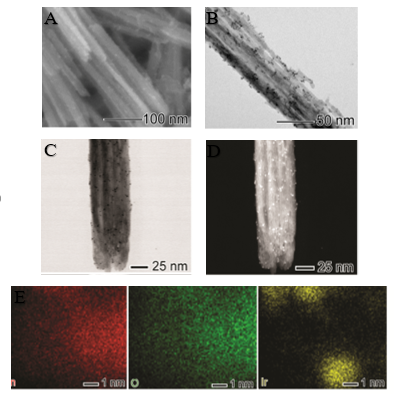
SEM images of MnO2-Ir nanowires (A) and TEM (B). BF- STEM (C) and HAADF-STEM (D) and STEM-EDS (E) maps of Mn (red), O (green), and Ir (yellow).
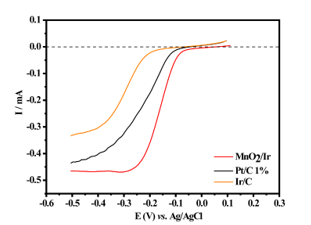
Polarization curves for the ORR on MnO2, MnO2-Ir,and 1.2 wt.% Pt/C materials, in 0.1 mol L−1 KOH solution, f = 1600 rpm in room temperature.
Conclusões
In conclusion, the present investigation demonstrated that MnO2 nanowires
decorated with Ir NPs could be employed as heterogeneous electrocatalysts for the
oxygen reduction reaction, being a promising nanocatalyst compared to commercial
platinum. Through a simple synthesis, it was possible to obtain nanowires with
defined shape and size that could serve as templates for Ir NPs nucleation and
growth without any surface modification, which oxidize to IrO2 while MnO2 is
reduced.
Agradecimentos
Referências
[1] Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538.
[2] Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The Recent Progress and Future of Oxygen Reduction Reaction Catalysis: A Review. Renew. Sustain. Energy Rev. 2017, 69, 401–414.
[3] Xia, Y.; Yang, X. Toward Cost-Effective and Sustainable Use of Precious Metals in Heterogeneous Catalysts. Acc. Chem. Res. 2017,50, 450–454.
[4] Goswami, C.; Hazarika, K.K.; Bharali, P. Transition Metal Oxide Nanocatalysts for Oxygen Reduction Reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128
[5] Cheng, F.; Su, Y.; Liang, J.; Tao, Z.; Chen, J. MnO2-Based Nanostructures as Catalysts for Electrochemical Oxygen Reduction in Alkaline Media. Chem. Mater. 2010, 22, 898–905.
[6] Wang, Z.; Gao, W.; Xu, Q.; Ren, X.; Xu, S.; Zhu, S.; Niu, X.; Li, X.; Zhao, R.; Han, Y.; et al. Influence of the MnO2 Phase on Oxygen Evolution Reaction Performance for Low-Loading Iridium Electrocatalysts. ChemElectroChem 2021, 8, 418–424.
















