Autores
Gallego-londoño, V. (UNIVERSIDAD DE ANTIOQUIA) ; Santa-gonzález, G.A. (INSTITUTO TECNOLÓGICO METROPOLITANO) ; Manrique-moreno, M. (UNIVERSIDAD DE ANTIOQUIA)
Resumo
Bioactive peptides (BAPs) are well-recognized for their broad spectrum of
applications including antitumoral activity due to their high cytotoxic action,
selectivity, and limited scope for drug resistance. In the present study, the
antitumoral activity of the synthetic peptides Ctn and NA-CATH-ATRA-1ATRA-1 was
evaluated against MCF-7 and MDA-MB-231 breast cancer cell lines. The flow
cytometry experiments showed an increase of the DiOC6(3) uptake and a
significant propidium iodide (PI) uptake at higher concentrations in both cell
lines. Additionally, it was possible to determine that peptides exert their
antitumoral activity by inducing cell cycle arrest. The results suggest that
these peptides could be considered potential therapeutic molecules in the
treatment of breast cancer.
Palavras chaves
Bioactive peptides (BAPs); antitumoral activity; breast cancer
Introdução
Bioactive peptides (BAPs) are considered part of the innate immune system of
several organisms and participate in the first line of defense in response to
the attack of pathogens (HANEY, MANSOUR, et al., 2017, TORNESELLO, BORRELLI, et
al., 2020). They have been extensively studied as a promising alternative to
antibiotics (HANCOCK, SAHL, 2006). However, their spectrum of activity extends
to parasites, fungi, and in the last years cancer cells (TORNESELLO, BORRELLI,
et al., 2020). The selectivity of these compounds for malignant over normal
cells is based on the electrostatic attraction for the negatively charged
molecules on cancer cell membranes such as phosphatidylserine phospholipids, O-
glycosylated mucins, and sialic acid-derived gangliosides (BAXTER, LAY, et al.,
2017). An interesting property of peptides is that most of them mediate
anticancer effects irrespective of the genetic and epigenetic features of
malignant cells, largely reflecting unique physiochemical properties that enable
them to interact and disrupt lipid bilayers (VITALE, YAMAZAKI, et al., 2021).
This is significantly important in a type of cancer with high intratumoral
heterogeneity such as breast cancer (LÜÖND, TIEDE, et al., 2021). In this study,
the Crotalicidin (Ctn) a peptide identified in Crotalus durissus (FALCAO,
DE LA TORRE, et al., 2014) and NA-CATH-ATRA-1-ATRA-1, which is a modification of
the NA-CATH peptide (DEAN, BISHOP, et al., 2011, ZHAO, GAN, et al., 2008) were
evaluated against MCF-7 and MDA-MB-231 cancer cell lines. Both peptides showed
promising activity against the cancer cell lines. As a control, the peptide LTX-
315 was used to compare the activity of the peptides.
Material e métodos
Peptides were synthesized by the solid-phase method (GUZMÁN, BARBERIS, et al.,
2007), and purchased from GenScript (Piscataway Township, NJ, USA). The purity
of the peptides was determined to be higher than 95% by analytical HPLC-UV, and
the molecular weight (MW) was confirmed with LC-ESI-MS. The LTX-315 peptide was
used as a positive control because it has been studied in the phase I trial in
patients with advanced solid tumors including breast tumors and demonstrates
being clinically active (SPICER, MARABELLE, et al., 2021). The cytotoxic
activity of the peptides against MCF-7 (ATCC HTB-22) and MDA-MB-231 (ATCC CRM-
HTB-26) cell lines was determined by a colorimetric 3-( 3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability assay after 24h of
treatment. The antitumor activity of the peptides was evaluated at
concentrations below the IC50 value obtained. 1 x 105cells
were grown into a 24-well plate and incubated with different peptide
concentrations for 6h. The following parameters were evaluated by flow
cytometry: (a) disruption of cell membrane integrity by propidium iodide (PI)
uptake; (b) change in mitochondrial membrane potential monitored through the
lipophilic cationic fluorochrome lipophilic 3-3'-dihexyloxacarbocyanine iodide
(DiOC6(3)); and (c) distribution of cell cycle phases by staining of
cells with PI. GraphPad Prism Software (Version 8.0.1, GraphPad Inc, CA, USA)
was used to perform data analysis. A one-way/two-way analysis of variance
(ANOVA) with post hoc comparisons via Fisher’s Least Significant Difference
(LSD) test was used to evaluate the level of significance (expressed as the p-
value).
Resultado e discussão
The physicochemical properties of the peptides are shown in Table 1. The results
of the cytotoxic activity showed that Ctn and NA-CATH-ATRA-1-ATRA-1 are more
active toward MDA-MB-231 cell line, with an IC50 value approx. of 2.1
to 2.8 times lower that the concentration needed for MCF-7 cells. The higher
cytotoxic activity of the peptides against MDA-MB-231 cell line could be
associated with differences in the cell-membrane composition (VITALE, YAMAZAKI,
et al., 2021). The flow cytometry results showed that both peptides induced an
increase in the percentage of PI-positive cells at the highest concentration
evaluated (Figure 1). However, when the peptides are used at lower
concentrations than those required for their membranolytic effect, they can
translocate to the cytosol and affect intracellular targets (VITALE, YAMAZAKI,
et al., 2021). In this study, it was observed that at subtoxic concentrations of
the peptides, induced an increase of the DiOC6(3) mean fluorescence
intensity (MFI) (Figure 1), which suggested that high trans-membrane potential,
known as mitochondria hyperpolarization, was involved. Investigations in breast
cell lines have suggested that cell cycle arrest is a common mechanistic marker
for peptide anticancer activity (MANRIQUE-MORENO, SANTA-GONZÁLEZ, et al., 2021).
All peptides in MCF-7 cells exerted an increase in the percentage of cells in
the G0/G1 with increasing peptide concentrations, which is concomitant with a
G2/M decrease (from 30.15% in untreated cells to 4.63-9.84% at the high
concentration of peptides). In contrast, cell cycle distribution of MDA-MB-231
was characterized by the accumulation of the cell population in the S phase
(from 33.87% in untreated cells to 45.03-69.88% at the highest concentration)
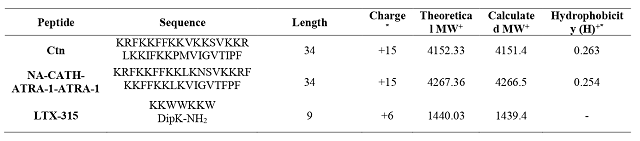
*Calculated with the ‘Peptide property calculator’ software (http://pepcal.com) +Molecular weight (MW) *+Calculated from http://heliquest.ipmc.cnrs.fr
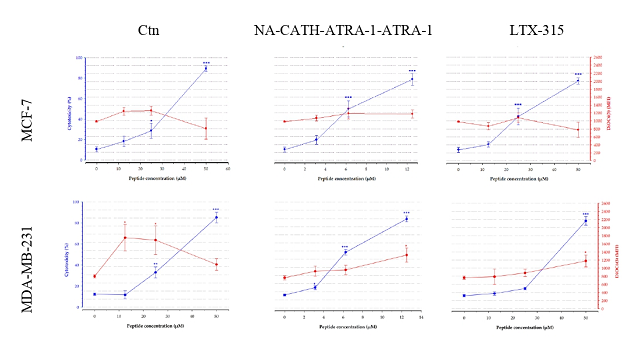
PI-positive cells (%) on the left axis (blue line). Mitochondrial membrane polarization was evaluated with DiOC6(3)(MFI)on the right axis (red line)
Conclusões
Ctn and NA-CATH-ATRA-1-ATRA-1 peptides showed the highest cytotoxic activity
towards MDA-MB-231 than MCF-7 cells. These peptides are more active than LTX-315
peptide control at MDA-MB-231 cell line. At lower peptide concentrations both cell
lines displayed an increase in mitochondrial membrane potential and cell cycle
arrest. The results suggest that Ctn and NA-CATH-ATRA-1-ATRA-1 are potential
target-studied molecules for breast cancer treatment. More studies are needed to
search the mechanism of action involved in their antitumoral effect.
Agradecimentos
This research was supported by MinCiencias Research Grant [Cod. 111584467189, RC
946-2019].
Referências
AHMED, S., MIRZAEI, H., ASCHNER, M., et al. "Marine peptides in breast cancer: Therapeutic and mechanistic understanding", Biomedicine and Pharmacotherapy, v. 142, p. 112038, 2021. DOI: 10.1016/j.biopha.2021.112038. Disponível em: https://doi.org/10.1016/j.biopha.2021.112038.
BAXTER, A. A., LAY, F. T., POON, I. K. H., et al. "Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects", Cellular and Molecular Life Sciences, v. 74, n. 20, p. 3809–3825, 2017. DOI: 10.1007/s00018-017-2604-z.
DEAN, S. N., BISHOP, B. M., VAN HOEK, M. L. "Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus.", BMC microbiology, v. 11, n. May, p. 114, 2011. DOI: 10.1186/1471-2180-11-114.
FALCAO, C. B., DE LA TORRE, B. G., PÉREZ-PEINADO, C., et al. "Vipericidins: A novel family of cathelicidin-related peptides from the venom gland of South American pit vipers", Amino Acids, v. 46, n. 11, p. 2561–2571, 2014. DOI: 10.1007/s00726-014-1801-4. .
GUZMÁN, F., BARBERIS, S., ILLANES, A. "Peptide synthesis: chemical or enzymatic", Electronic Journal of Biotechnology, v. 10, n. 2, p. 279–314, 2007. DOI: 10.2225/vol10-issue2-fulltext-13.
HANCOCK, R. E. W., SAHL, H. G. "Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies", Nature Biotechnology, v. 24, n. 12, p. 1551–1557, 2006. DOI: 10.1038/nbt1267.
HANEY, E. F., MANSOUR, S. C., HANCOCK, R. E. W., "Antimicrobial peptides: an introduction". In: HANSEN, P. R. (Org.), Antimicrobial peptides. Methods and Protocols., Methods in ed. [S.l.], Humana Press Inc., 2017. p. 3–22. DOI: 0.1007/978-1-4939-6737-7.
LÜÖND, F., TIEDE, S., CHRISTOFORI, G. "Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression", British Journal of Cancer, v. 125, n. 2, p. 164–175, 2021. DOI: 10.1038/s41416-021-01328-7. Disponível em: http://dx.doi.org/10.1038/s41416-021-01328-7.
MANRIQUE-MORENO, M., SANTA-GONZÁLEZ, G. A., GALLEGO, V. "Bioactive cationic peptides as potential agents for breast cancer treatment", Bioscience Reports, v. 41, n. 12, 2021. DOI: 10.1042/BSR20211218C.
SPICER, J., MARABELLE, A., BAURAIN, J.-F., et al. "Safety, antitumor activity, and T-cell responses in a dose-ranging phase I trial of the oncolytic peptide LTX-315 in patients with solid tumors", Clinical Cancer Research, v. 27, n. 10, p. 2755–2763, 2021. DOI: 10.1158/1078-0432.ccr-20-3435.
TORNESELLO, A. L., BORRELLI, A., BUONAGURO, L., et al. "Antimicrobial peptides as anticancer agents: Functional properties and biological activities", Molecules, v. 25, n. 12, p. 1–26, 2020. DOI: 10.3390/molecules25122850.
VITALE, I., YAMAZAKI, T., WENNERBERG, E., et al. "Targeting cancer heterogeneity with immune responses driven by oncolytic peptides", Trends in Cancer, v. 7, n. 6, p. 557–572, 2021. DOI: 10.1016/j.trecan.2020.12.012. Disponível em: https://doi.org/10.1016/j.trecan.2020.12.012.
ZHAO, H., GAN, T. X., LIU, X. D., et al. "Identification and characterization of novel reptile cathelicidins from elapid snakes", Peptides, v. 29, n. 10, p. 1685–1691, 2008. DOI: 10.1016/j.peptides.2008.06.008.
















