Autores
García Maza, L. (GIQOBIO, UNIVERSIDAD DEL ATLÁNTICO) ; Orosco Florez, D. (GIQOBIO, UNIVERSIDAD DEL ATLÁNTICO) ; Mendoza Salgado, A. (GIQOBIO, UNIVERSIDAD DEL ATLÁNTICO) ; Rosales Rada, W. (GIAB, UNIVERSIDAD LIBRE - SECCIONAL BARRANQUILLA) ; Mendoza Torres, E. (GIAB, UNIVERSIDAD LIBRE - SECCIONAL BARRANQUILLA) ; Meléndez Gómez, C. (GIQOBIO, UNIVERSIDAD DEL ATLÁNTICO)
Resumo
Gram-negative bacteria such as Escherichia coli (E. coli) can
exhibit resistance to multiple commonly used drugs. Thus, compounds that are
active against persistent bacteria are needed. Tetrahydroquinoline core appears
as a study strategy as potential antimicrobial agent owing to their different
biological applications. Hence, in this work, we synthesize a series of
N-(2-(o-tolyl)-1,2,3,4-tetrahydroquinoline-4-yl)formamide
derivatives using sequential acid catalyzed imino Diels-Alder methodology with
moderate yields. The bacterial activity of these tetrahydroquinolines was
evaluated against E. coli. 5a-f were determined using the Minimum
Inhibitory Concentration (MIC), showing important activity values as the
molecules 5a, 5c, and 5f, (MIC = 10 µg/mL).
Palavras chaves
Synthesis; Antibacterial activity; Tetrahydroquinoline
Introdução
Antibiotic resistance continues to threaten public health, and multidrug-
resistant pathogens have emerged with enormous economic costs to healthcare
systems (DAYAL et al., 2019). In addition, gram-negative bacteria, as etiologic
agents such as Escherichia coli, have emerged as an important pathogen,
due to their ability to generate antimicrobial resistance processes increase.
The rise of the mechanisms of microbial resistance to existing drug therapies
reveals the need for new and efficient therapeutic agents (ROSSOLINI et al.,
2014). Consequently, there is an urgent need to develop novel antibacterial
agents with potent activity against drug-resistant strains. Heterocyclic
compounds are distributed in nature and are broadly used in medicinal chemistry
(KADHIM; KHAMMAS, 2014). Tetrahydroquinoline (THQ) and their synthetic
derivatives have emerged with remarkable pharmacological properties, such as
antimicrobial, antiviral and antibiotic agents (MANOLOV et al., 2021).
Therefore, the synthesis of THQs is described extensively in the literature,
focusing on the search for a general, simple, efficient, and low-cost
preparation method. The growing interest can be explained by their biological
activities and synthesis methodologies for preparing tetrahydroquinoline
derivatives (MUTHUKRISHNAN et al., 2019). Keeping the view of the biological
importance of heterocyclic compounds (CHAVAN et al., 2019), we studied the
biological activity of synthetic tetrahydroquinoline derivatives as
antibacterial agents. The purpose of our work was to synthesize N-(2-
(o-tolyl)-1,2,3,4-tetrahydroquinoline-4-yl)formamide derivatives
exploring the versatility of the iDA process and evaluate their biological
activities as potential growth inhibitors against E. coli.
Material e métodos
Chemistry. The substituted N-(2-(o-tolyl)-1,2,3,4-
tetrahydroquinoline-4-yl)formamide (5a-f) were synthesized through a sequential
imine-Diels-Alder (iDA) reaction; the N-phenyl-2-(o-
tolyl)methanimines (3a-f) were obtained by the condensation reaction of the
available diverse anilines (1a-f) (1.0 mmol) and o-tolualdehyde 2 (1.0
mmol) in ethanol (10 mL) at room temperature (r.t.). Then, the desired
tetrahydroquinoline 5a-f were synthesized via the cycloaddition reaction between
the N-phenyl-2-(o-tolyl)methanimine precursors 3a-f and N-
vinyl formamide 4 in MeCN (20 mL) using p-TsOH (10% mol) at r.t. To
afford the desired products, the crude mixture was purified by column
chromatography (silica gel, Hexane:ethyl acetate). Biological activity.
Antibacterial activity was determined using the two-fold serial broth dilution
method according to the guidelines of the clinical laboratory standards
institute (CLSI) (DAYAL et al., 2019). 10 µL of a 0.5 McFarland culture (LOZANO
et al., 2018) was grown on 200 µL of Mueller Hinton medium with several
concentrations (10, 20, 35, 50 y 75 µg/mL) of each of the synthetic compounds.
Absorbance was measured at 660 nm every hour for 8 hours. Each concentration of
the compounds was performed in duplicate for (n=3). Positive inhibition control
(Gentamicin/20 µg/mL), cytotoxicity (DMSO 1%), and growth control consisting of
compound-free bacterial culture (a microbial strain of Escherichia coli
(ATCC 25922) were used. The percentage of inhibition was calculated with the
Excel program. And One-way Analysis of Variance (ANOVA) was applied with the
Tukey post-test. The figures were made with GraphPad prism version 8.0.1.
Resultado e discussão
The N-(2-(o-tolyl)-1,2,3,4-tetrahydroquinoline-4-yl)formamide (5a-
f) was obtained with moderate yields through a sequential iDA reaction, using
p-TsOH as a catalyst (Scheme 1). The results are according to the
previously described compounds (HERNÁNDEZ, 2019). IR spectra revealed absorption
bands at 3240–3667 cm-1 characteristic for the N-H groups and 1667
cm-1 attributed to the amide group (C=O stretching). Characteristic
signal of 1H-NMR showed aromatic protons at 6.53–7.56 ppm, a singlet
(s) of three protons at 2.26 ppm for the o-CH3 group, a (s) in
3.89 ppm due to NH group, a doublet (d) at 5.80-5.82 ppm (J = 9.2 Hz) for the
4NH(CO) group and an (s) at 8.29 ppm for the (CO)H group, as well as a multiplet
(m) at 1.81-1.90 ppm and a doublet of doublets (dd) at 2.42-2.45 ppm (J = 6.1,
2.5 Hz) for the CH2 group, a doublet of doublets of doublets (ddd) at
5.55-5.61 ppm (J = 10.0, 9.9, 6.2 Hz) and a (dd) at 4.77-4.80 ppm (J = 10.9, 2.5
Hz) for the tetrahydroquinoline moiety group. Furthermore, they were also
supported by their 13C NMR and two-dimensional NMR experiments. The
activity of these compounds follows the trend reported in previous studies
(FAIDALLAH et al., 2014; PHAM et al., 2019; MANOLOV et al., 2021) with moderate
growth inhibition. Derivatives with halogenated substitutes at the C-6 position
show higher percentages of inhibition compared to methyl-type substitutes
(F>I>Cl>Me>isopropyl>H). However, no activity was found by N-(2-
(o-tolyl)-1,2,3,4-tetrahydroquinoline-4-yl)formamide (5e), even to the
highest concentration (75 µg/mL). The lower MIC values were obtained by the
halogenated substitutes (5a, 5f) against E. coli (10 µg/mL) (Figure 1).
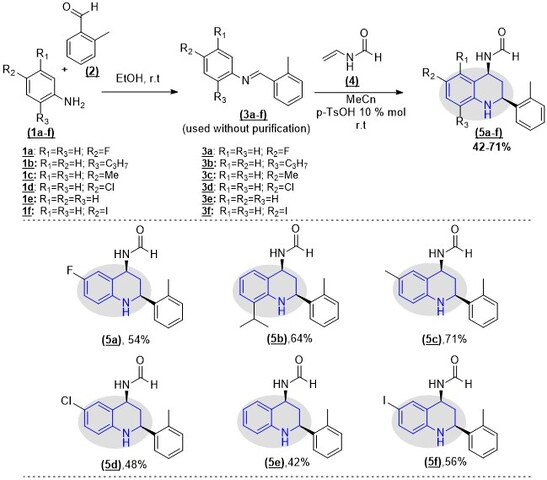
Synthetic route for obtaining N-(2-(o- tolyl)-1,2,3,4-tetrahydroquinoline-4-yl)formamide derivatives through tandem sequential iDA reaction.

a) growth inhibition of E. coli; b) MIC.
Conclusões
Sequential iDA reaction continues to be one of the main cycloaddition strategies
for synthesizing tetrahydroquinoline derivatives. Compounds with electron-
withdrawing groups could be considered growth inhibitors, with MIC of 10 µg/mL
that deserves further investigation to explore the scope and limitation of their
biological activities. In addition, detailed studies are being carried out to
associate the results obtained in this research with more precise information on
the mechanisms of action of these molecules.
Agradecimentos
L.J.G.M and D.F.O.F thank to Ministerio de Ciencia Tecnología e Innovación
(CTeI;519-2021 from 874-2020) for the financial support.
Referências
CHAVAN, Pravin N. et al. Synthesis and biological activities of new tetrahydroquinoline and pyrimidine derivatives. European Chemical Bulletin, v. 8, n. 8, p. 257–264, 2019.
DAYAL, Neetu et al. Inhibitors of Intracellular Gram-Positive Bacterial Growth Synthesized via Povarov-Doebner Reactions. ACS Infectious Diseases, v. 5, n. 11, p. 1820–1830, 2019.
FAIDALLAH, Hassan M. et al. Synthesis and biological evaluation of some novel tetrahydroquinolines as anticancer and antimicrobial agents. Journal of Enzyme Inhibition and Medicinal Chemistry, v. 29, n. 3, p. 367–378, 2014.
HERNÁNDEZ, Mayra. SÍNTESIS DE NUEVAS 2-ARIL-TETRAHIDROQUINOLINAS SUSTITUIDAS VÍA REACCIÓN iDA MULTICOMPONENTE. 2019. 2–41 f. Univerisdad del Atlántico, 2019.
KADHIM, Mohammed A; KHAMMAS, Sameaa J. Synthesis and biological evaluation of some tetrahydroquinoline compounds derived from 4-Aminobenzenesulfonamid. J. of university of Anbar for pure science, v. 8, n. 1, p. 4–8, 2014.
LOZANO, Guzmán Eduardo et al. Low accuracy of the McFarland method for estimation of bacterial populations. African Journal of Microbiology Research, v. 12, n. 31, p. 736–740, 2018.
MANOLOV, STANIMIR P.; IVANOV, ILIYAN I.; BOJILOV, DIMITAR G. Microwave-assisted synthesis of 1,2,3,4-tetrahydroisoquinoline sulfonamide derivatives and their biological evaluation. Journal of the Serbian Chemical Society, v. 86, n. 2, p. 139–151, 2021.
MUTHUKRISHNAN, Isravel; SRIDHARAN, Vellaisamy; MENÉNDEZ, J. Carlos. Progress in the Chemistry of Tetrahydroquinolines. Chemical Reviews, v. 119, n. 8, p. 5057–5191, 2019.
PHAM, Thu D.M.; ZIORA, Zyta M.; BLASKOVICH, Mark A.T. Quinolone antibiotics. MedChemComm, v. 10, n. 10, p. 1719–1739, 2019.
ROSSOLINI, Gian Maria et al. Update on the antibiotic resistance crisis. Current Opinion in Pharmacology, v. 18, p. 56–60, 2014.
















