Autores
Rodriguez Ibañez, D.F. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Lipez Pinzón, K.J. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Ardila Rodriguez, D.M. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Vera Alarcón, D.R. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Alvarez, G. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Palma Rodriguez, A. (UNIVERSIDAD INDUSTRIAL DE SANTANDER) ; Cobo Domingo, J. (UNIVERSIDAD DE JAEN) ; Nogueras Montiel, M. (UNIVERSIDAD DE JAEN)
Resumo
The quinazoline motif have long been recognized as a privilege scaffold in medicinal chemistry. In order to
expand its molecular diversity and evaluate the potential biological manifestations of 4-styrylquinazoline
derivatives, we have developed a facile and straightforward one-pot methodology based on the copper-
mediated coupling of 2’-aminophenylchalcones 1 with aromatic aldehydes 2 and ammonium acetate using
chlorobenzene as solvent to obtain, in good yields and in short reaction times, the novel 2-aryl-4-
styrylquinazolines 3.
Palavras chaves
4-styrylquinazolines; 2’-aminophenylchalcones; oxidative-cyclization
Introdução
The quinazoline derivatives are of interest in medicinal chemistry and pharmacology. These compounds have
attracted great attention for their promising application as therapeutic agents in view of their biological
activity, for example as anticancer, anti-inflammatory, antibacterial, antivirus, and antipsychotic agents
(ELHAM et al, p. 1, 2016). The functionalization of quinazoline ring is an efficient strategy to enhance its
pharmacological activity and/or offer more interesting properties. Consequently, it is not surprising that a
significant number of synthetic methodologies have been developed for the synthesis of new derivatives of
this heterocyclic system (WU et al, p. 672, 2020). However, regarding the synthesis of 4-styryl-2-
arylquinazolines, only few reports are encountered in literature, and, in most of cases, complex starting
compounds (YAO et al, p. 168, 2015), or expensive metal-based catalysts and strong bases with cumbersome
handling are used (DAN et al, p. 5908, 2013), even long reaction times are attained when 2’-
aminophenylchalcones are used as precursors (GANDHESIRI et al, p. 4774, 2019). With the aim to explore the
synthetic utility of 2’-aminophenylchalcones and overcome these difficulties, we have successfully developed
an alternative methodology based upon the copper acetate/acetic acid promoted three-component oxidative-
cyclization of 2’-aminophenylchalcones with aromatic aldehydes and ammonium acetate as nitrogen source,
using chlorobenzene as solvent.
Material e métodos
The 2’-aminophenylchalcones 1a-h, aromatic aldehydes 2a-e, ammonium acetate and copper acetate were
mixed in sealed flasks with 2.0 mL of an acetic acid-chlorobenzene mixture (Scheme 1). The reaction mixtures
were then heated at 150 C for 1-3 h. The reaction media was washed using distilled water and extracted with
dichloromethane (3 x 20 mL). Then, the combined organic phase was dried over sodium sulphate anhydrous,
concentrated under reduced pressure and the organic residue purified by column chromatography over silica
gel using n-heptane-ethylacetate as eluent (10:1 – 5:1).
Resultado e discussão
As shown in Scheme 1, the reaction of 1, 2 and ammonium acetate in presence of a slight excess of copper
acetate and catalytic amounts of acetic acid produced the target 2-aryl-4-styrylquinazolines 3 as exclusive
products. After several preliminary experiments, we found that the most suitable reactions times and
temperatures to reach the desired styrylquinazolines 3 were 1-3 h and 150 °C, respectively. Even though the
consumption of the starting substrates was complete as was evidenced by TLC, the isolated yields of 3
ranged from moderate to good (70-85%), essentially affected by the nature and positions of the substituents
on the benzene ring at C-2 position. All the quinazolines 3 were isolated as solids with well-defined melting
points and further fully characterized by IR, 1H/13C NMR and HR-MS.
Additionally, all the synthesized compounds were proposed and sent to the National Cancer Institute (NCI) of
the USA for their antiproliferative evaluation over a 60-panel cancer cell lines (currently undergoing).
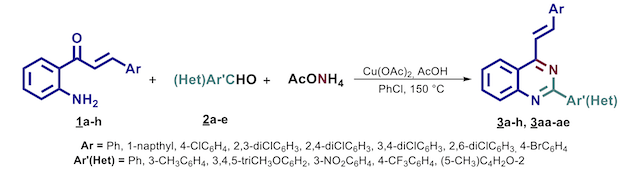
One-pot three-component synthesis of 2-aryl-4-styrylquinazolines 3 via copper mediated oxidative cyclization of 2’- aminophenylchalcones.
Conclusões
The methodology described in this work constitutes an alternative, facile and straightforward route to access
novel 2-aryl-4-styrylquinazolines, via a one-pot copper mediated oxidative-cyclization of synthetically
available 2’-aminophenylchalcones with aromatic aldehydes and ammonium acetate.
Agradecimentos
Authors acknowledge the finantial support to Vicerrectoría de Investigación y Extensión of the Universidad
Industrial de Santander.
Referências
DAN, Z.; QI, S.; YU-REN, Z.; JIAN-XIN, L. KOtBu-Mediated Stereoselective Addition of Quinazolines to Alkynes under Mild Conditions. Organic & Biomolecular Chemistry, n. 11, 5908-5912, 2013.
GANDHESIRI, S.; POLU, A.; LAXMAN, K.; ANDIVELU, I. Copper‐Catalyzed Oxidative Amination of Methanol to Access Quinazolines. Organic and Biomolecular Chemistry, n. 17, 4774-4782, 2019.
ELHAM, J.; MARZIEH, R.; FARSHID, H.; GHOLAM, H.; GHADAM, A. Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Research in Pharmaceutical Sciences, n. 11, 1-14, 2015.
YAO, C.; GAWANDE, S.; ZANWAR, M.; KAVALA, V.; KUO, R. One-Pot Synthesis of 2-Arylquinazolines and Tetracyclic Isoindolo[1,2-a]quinazolines via Cyanation Followed by Rearrangement of ortho-Substituted 2-Halo-N-arylbenzamides. Advanced Synthesis and Catalysis, n. 1, 168-176, 2015.
YUNHE, L.V.; YAN, L.; TAO X.; WEIYA, P.; HONGWEI Z.; KAI S.; QUN, L.; QIAN, Z. Copper-catalyzed annulation of amidines for quinazoline synthesis. Chemical Communications, n. 49, 6439-6441, 2013.
WU, Y.; HAI, L.; XING, H.; CHEN, J.; SHI, Y.; HUANG, T. Synthesis of 4-ethenyl Quinazolines via Rhodium(III)-Catalyzed [5+1] Annulation Reaction of N-arylamidines with Cyclopropenones. Organic Chemistry Frontiers, n. 7, 672-677, 2020.
















