Autores
dos Santos Filho, J.M. (UFPE) ; Pinheiro, S.M. (UFPE) ; Gomes da Silva, M. (UFPE) ; Siqueira Silva, L.V. (UFPE)
Resumo
The building blocks of proteins, hormones, toxins, neurotransmitters, and nucleic
bases are the amino acids, amazing simple molecules. Despite their central role
in structuring life, the main core of the essential α-amino acids includes only 20
molecules. These remarkable substances are easily recognized, absorbed, and
metabolized by living beings, whose complexity is based upon them. Even
structurally modified amino acids are important in the metabolic pathways since
they remain recognizable to the biomolecules ruling the cells. Therefore, it is
reasonable to suppose that modifications at the amino acid backbone aiming to
incorporate bioactive pharmacophores and privileged structures can lead to new and
structurally diverse drugs.
Palavras chaves
Thiosemicarbazides; 2,4-DNP-phenylalanine; Antitumor activity
Introdução
Amino acids (AA) are the basic units of various biomolecules found in living
beings, especially proteins. Therefore, they are easily recognized by the
biomolecules regulating cell processes, aside from being the starting materials
for the biosynthesis of several bioactive small molecules, essential for
life's maintenance and regulation. For example, phenylalanine (Phe) is the
precursor of tyrosine (Tyr), which is converted to catecholamines, a group of
three substances comprising dopamine, norepinephrine, and epinephrine, which
exert effects as either a neurotransmitter or as a hormone in numerous parts of
the body. Methionine (Met) is essential for building the cofactor S-adenosyl
methionine, an important methyl donor in some biological processes, while
histidine (His) leads to histamine, a potent neurotransmitter. All other AA are
of similar importance for the biosynthesis of regulatory or structural
biomolecules [KAMBLE et al, 2021]. Due to their biological responses, AA
are frequently hybridized with several privileged structures and/or well-known
pharmacophoric groups looking for the discovery of new bioactive compounds [XU
et al, 2021]. Promising investigations in this field can
be easily found in the literature, especially for antitumor activities [DE
CASTRO et al, 2020]. One of the most ancient AA modifications is of
central importance for the development of the synthesis and structural
elucidation of peptides, consisting of the attachment of the amino group to the
2,4-dinitrophenyl (DNP) moiety via a SNAr reaction, leading to
Nα-2,4-dinitrophenyl amino acids (DNP-AA) [ SANGER, 1945]. The
nitroaromatic portion found in many compounds with great therapeutic
significance is essential to the biological activity so that its removal leads
to the potency’s loss or even the complete lack of pharmacological response
[NEPALI et al, 2019]. Despite the DNP-AA having been known for a long
time in the literature, no investigation of their biological properties has been
carried out until this point. On that account, dinitrophenyl phenylalanine (DNP-
Phe) derivatives synthesis is a relatively simple process, whose isolation and
purification are quite easy, allowing their preparation as pure products. The
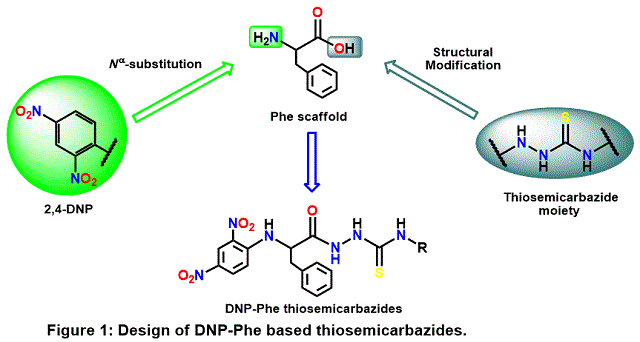
chosen strategy for this work has envisaged the design of derivatives bearing
the thiosemicarbazide moiety due to its importance in medicinal chemistry,
particularly as antitumor bioactive molecules [ACHARYA et al, 2021].
Several DNP-Phe thiosemicarbazide derivatives were designed, synthesized, and
characterized, and the compounds DNP-Phe(1-13) are expected to exhibit
antitumoral responses. The structural Phe modification design is depicted in
Figure 1.
Material e métodos
Melting points were determined with a capillary apparatus and are uncorrected.
The progress of the reactions was monitored by thin-layer chromatography (TLC),
performed onto glass-backed plates of silica gel 60 F254 with gypsum from Merck,
and all compounds were detected by ultraviolet light (254 nm). Nuclear magnetic
resonance (NMR) spectra were recorded at 400 MHz for hydrogen (1H)
and 100 MHz for carbon-13 (13C). Analyses were determined at 25 °C in
DMSO-d6 with chemical shift values (δ) in parts per million (ppm)
and coupling constants (J) in Hertz (Hz). 1H NMR and 13C
NMR assignments were assisted by 2D experiments. Infrared (IR) spectra were
recorded on an FTIR spectrometer from Bruker with the samples being analyzed as
KBr pellets. Elemental analyses were performed in a Perkin Elmer elemental
analyzer.
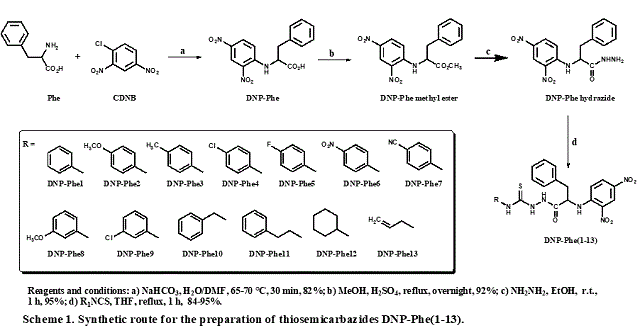
The synthesis of the substituted thiosemicarbazides DNP-Phe(1-13), depicted in
Scheme 1, was based on the structural modifications of the commercially
available amino acid phenylalanine (Phe) as starting material. A nucleophilic
aromatic substitution (SNAr) reaction between Phe and 1-chloro-2,4-
dinitrobenzene (CDNB) was accomplished, leading to the N
α-(2,4-dinitrophenyl)-phenylalanine (DNP-Phe) under appropriate
conditions in good yield and high purity of the crude product. Afterward, a
simple Fisher esterification of compound DNP-Phe in methanol and catalyzed by
mineral acid has led to the corresponding DNP-Phe methyl ester. The key DNP-Phe
hydrazide was readily prepared in presence of hydrazine hydrate and short
reaction time. The DNP-Phe hydrazide has undergone a smooth addition reaction
with suitable isothiocyanates in THF under mild conditions, leading to the
thiosemicarbazide series DNP-Phe(1-13). Once all experiments were concluded, the
pure products were confirmed using IV and elemental analysis, as well as by NMR
spectroscopy. After confirming the purity, all compounds were submitted to the
biological evaluation of their antitumor activity.
Resultado e discussão
The synthetic route depicted in Scheme 1 was successfully carried out, starting
from the SNAr reaction between Phe and CDNB, which has led to the
DNP-Phe in 79% yield. The Fischer esterification has introduced the next
modification, also with an excellent outcome after a simple work-up, giving the
DNP-Phe methyl ester with 88% yield. The key intermediate DNP-Phe hydrazide has
been also obtained as a yellow solid and 92% yield of the crude product. The
thiosemicarbazides DNP-Phe(1-13) have been readily prepared under mild
conditions by reacting DNP-Phe hydrazide with appropriate isothiocyanates, in
order to introduce a diversity of substituents in the series. Such substituents
can help the biological evaluation and the establishment of the structure-
activity relationship (SAR) arising from the biological results. After
isolation, structural characterization of pure products DNP-Phe(1-13) was
carried out, confirming the planned chemical structures, as follows.
DNP-Phe1: Yield 90%; Mp 124.4-126.5 °C; Rf 0.57 (AcOEt); IR
(KBr, cm-1): 3327, 3175 (NH), 3105 (Ar CH ), 2999 (Aliphatic CH),
1708 (C=O), 1618 (C=C); DNP-Phe2: Yield 82%; Mp 128.9-130.4 °C;
Rf 0.58 (AcOEt); IR (KBr, cm-1): 3333, 3249, 3164 (NH),
2962 (Aliphatic CH), 1680 (C=O), 1619 (C=C); DNP-Phe3: Yield 83%; Mp
180.6-182.1 °C; Rf 0.67 (AcOEt); IR (KBr, cm-1): 3335,
3288, 3103 (NH), 3062 (Ar CH), 2924 (Aliphatic CH), 1686 (C=O), 1616 (C=C);
DNP-Phe4: Yield 76%; Mp 129.6-131.1 °C; Rf 0.45 (AcOEt); IR
(KBr, cm-1): 3319, 3104 (NH), 3027 (Ar CH ), 2954 (Aliphatic CH),
1695
(C=O), 1619 (C=C); DNP-Phe5: Yield 66%; Mp 137.2-138.9 °C; Rf
0.45 (AcOEt/MeOH 8:2); IR (KBr, cm-1): 3331, 3251, 3103 (NH), 3029
(Ar CH), 2967 (Aliphatic CH), 1682 (C=O), 1619 (C=C); DNP-Phe6: Yield
87%; Mp 147.1-150.0 °C; Rf 0.46 (AcOEt/MeOH 8:2); IR (KBr,
cm-1): 3328 (NH), 3099, 3029 (Ar CH ), 2956, 2921 (Aliphatic CH),
1618 (C=O), 1591 (C=C); DNP-Phe7: Yield 92%; Mp 142.2-143.7 °C;
Rf 0.47 (AcOEt/MeOH 8:2); IR (KBr, cm-1): 3322, 3101 (NH),
3028 (Ar CH), 2227 (C≡N), 1695 (C=O), 1617 (C=C); DNP-Phe8: Yield 82%; Mp
141.0-142.1 °C; Rf 0.65 (AcOEt/MeOH 8:2); IR (KBr, cm-1):
3334, 3280, 3105 (NH), 3028 (Ar CH), 2939 (Aliphatic CH), 1693 (C=O), 1618
(C=C); DNP-Phe9: Yield 81%; Mp 141.9-143.3 °C; Rf 0.57
(AcOEt/MeOH 9:1); IR (KBr, cm-1): 3334, 3103 (NH), 3027 (Ar CH ),
2965 (Aliphatic CH), 1680 (C=O), 1619 (C=C); DNP-Phe10: Yield 75%; Mp
218.5-220.2 °C; Rf 0.75 (AcOEt); IR (KBr, cm-1): 3346,
3289, 3102 (NH), 3027 (Ar CH ), 2934 (Aliphatic CH), 1695 (C=O), 1618 (C=C);
DNP-Phe11: Yield 76%; Mp 167.4-169.6 °C; Rf 0.67 (AcOEt); IR
(KBr, cm-1): 3363, 3331, 3255, 3177 (NH), 3027 (Ar CH ), 2970
(Aliphatic CH), 1698 (C=O), 1620 (C=C); DNP-Phe12: Yield 70%; Mp 173.7-
175.5 °C; Rf 0.63 (AcOEt); IR (KBr, cm-1): 3331, 2106
(NH), 3029 (Ar CH ), 2932, 2854 (Aliphatic CH), 1683 (C=O), 1618 (C=C); DNP-
Phe13: Yield 80%; Mp 185.9-187.4 °C; Rf 0.63 (AcOEt); IR (KBr,
cm-1): 3338, 3282, 3250, 3191 (NH), 3086 (Ar CH ), 2977 (Aliphatic
CH), 1692 (C=O), 1619 (C=C).
Conclusões
A series of N-(2,4-dinitrophenyl)-phenylalanine thiosemicarbazides DNP-Gly(1-13),
designed as potential antitumor molecules, was successfully prepared and
characterized by spectroscopic techniques after purification. The structural
modification of the phenylalanine scaffold incorporating the thiosemicarbazide
moiety has represented an outstanding strategy for the development of potentially
bioactive compounds, opening the possibility of discovering new lead molecules
with innovative structural features.
Agradecimentos
The authors are grateful to Mrs. Eliete de Fátima V. B. N. da Silva and the
Analytical Centre of Fundamental Chemistry Department, Universidade Federal de
Pernambuco, for the NMR, and IR experiments.
Referências
ACHARYA, P.T., BHAVSAR, Z.A., JETHAVA, D.J., PATEL, D.B., PATEL, H.D. A review on development of bio-active thiosemicarbazide derivatives: Recent advances, J. Mol. Struct. 1226 (2021) 129268.
DE CASTRO, P.P., SIQUEIRA, R.P., CONFORTE, L., FRANCO, C.H.J., BRESSAN, G.C., AMARANTE, G.W. Cytotoxic Activity of Synthetic Chiral Amino Acid Derivatives, J. Braz. Chem. Soc. 31 (1) (2020) 193-200.
KAMBLE, C., CHAVEN, R., KAMBLE, V. A Review on Amino Acids, Res. Rev. J. Drug Des.
Discov. 8 (3) (2021) 19-27.
NEPALI, K., LEE, H.-Y., LIOU, J.-P., Nitro-Group-Containing Drugs, J. Med. Chem. 62 (2019) 2851-2893.
SANGER, F., The Free Amino Groups of Insulin, Biochem. J. 39 (1945) 507-515.
Xu, Q., Deng, H., Li, X., Quan, Z.-S. Application of Amino Acids in the
Structural Modification of Natural Products: A Review Front. Chem. 9 (2021) 650569.
















