Autores
Martinez, M. (UNIVERSIDAD DE CALDAS) ; Parra, R.D. (DEPAUL UNIVERSITY) ; Ocampo-cardona, R. (UNIVERSIDAD DE CALDAS)
Resumo
1,3,5-polyols and more complex polyols of related structure become interesting
molecular models to study long-range proton transfers in probe molecules or
even in biological systems. Aiming to further study more complex long-range
proton transfers, and inspired in high-impact literature reports on long-range
“water-wires”- or “ammonium-wires”-mediated tautomerization processes, a proton-
transfer simple model was studied involving dimers of 1,3-propanediol or 1,3,5-
pentanetriol. With this model, the ability of an aliphatic polyol to facilitate
proton transfer is examined in dimers of 1,3-diols and dimers of 1,3,5-triols.
Also, looking ahead to attempt an experimental approach to prove this model,
1,3,5-pentanetriol was synthesized, and preparation of longer ‘skyped polyols’.
Palavras chaves
polyols; proton transfer ; long-range tautomerizn.
Introdução
Enzyme-catalyzed reactions often involve near or long-range proton transfers
(CUKIER, p. 337, 1998; BLOMBERG, p. 969, 2006), so scientists have been
encouraged to investigate them. For example, 1,5 tautomerization on 7-
hydroxyquinoline was studied in methanol solution (FANG, p. 7568, 1998), water-
chain clusters-mediated (LEUTWYLER, p. 381, 1999; ABOU-ZIED, p. 4195, 2011) or
involving ammonia clusters (LEUTWYLER, p. 11446, 2001; LEUTWYLER, p. 5933,
2003). As it is known that a ‘skyped’ heptaol networking dramatically enhance
acidity of a tertiary alcohol (KAS, et al., p. 10646, 2012), it would be
expected that polyols behave similar to water clusters. Inspired on this, a
preliminary theoretical study was performed on the diol-mediated intramolecular
proton transfer in glycine for its zwitterion/aminocarboxyl equilibrium (PARRA,
p. 33, 2019). So, the first part of our work involved the calculation of a
theoretical model for proton transfer in ‘skyped’ 1,3-diol or 1,3,5-triol
dimers.
On the other hand, it is well known that complex organic molecules containing a
‘skyped polyol’ moiety are useful as antifungal medicines or antibiotics, so
extensive studies have been performed to develop systematic methodologies to
prepare them (QUINTARD, p. 1025, 2020). Two broad methodologies are useful: (i)
successive aldol-type procedures which allow unidirectional growth of carbon
chains bearing the ‘skyped polyol’ moiety upon carbonyl reductions; (ii) two-
directional methodologies for the simultaneous head and tail growing of the
chain.
Aiming to further attempt experimental tests for theoretical the afore mentioned
model, the second part of this work involved the preparation of 1,3,5-
pentanetriol. Additionally, it was explored the synthesis of longer polyols.
Material e métodos
Organic synthesis of polyols. Dried solvents, precursors and reactants were
purchased from Aldrich. Intermediate synthetic and final products were
characterized by 1H-NMR and 13C-NMR (in a 500 MHz Bruker instrument at DePaul
University) and compared with literature. For the synthesis of 1,3,5-
pentanetriol, starting material was commercially available diethyl 1,3-
acetonedicarboxylate. Reductions were performed with NaBH4 or LiAlH4.
Dihydropyran and PTSA were used for protection and further deprotection of
hydroxyl group.
Synthetic sequence involved four steps: (1) reduction of diethyl 1,3-
acetonedicaroxylate with NaBH4/methanol; (2) acid-catalyzed DHP-protection of
the resulting secondary alcohol; (3) reduction of head and tail with LiAlH4 in
ether; (4) deprotection of the O-THP protected secondary hydroxyl group. The
product was dried by azeotropic distillation.
Exploration of the synthesis of a larger polyol: (i) 1,3,5-pentanetriol was
protected, and the primary non-protected alcohol was converted into the
respective aldehyde; (ii) a Reformatsky reaction was performed with ethyl
bromoacetate followed by with DHP/PTSA protection; (iii) LAH reduction and final
deprotection should afford 1,3,5,7-heptanetetraol.
Computational methods. Optimizations, frequency calculations, and single-point
energy calculations for all systems were performed with the B3LYP/6-31+G(d,p)
level of theory using the Gaussian 16 package of programs. Minimum energy
structures were confirmed by the absence of any imaginary frequencies, while
transition state structures by the presence of one imaginary frequency. IRC
calculations further confirm the connection between the transition state
structures and corresponding minima.
Resultado e discussão
Synthesis of the triol. Synthetic sequence is depicted in scheme 1, adapted from
the literature (MORI, p. 45, 1987). NaBH4 reduction of starting material,
followed by PTSA/DHP gave rise to the respective THPO-protected secondary
alcohol. Treatment of this product with LiAlH4, followed by TPSA/methanol
deprotection gave rise to the expected product. NMR data in ppm (500 MHz, D2O)
agree with literature (WENDER et al., p. 13648, 2002): 1.68 (4H, m), 3.66 (4H,
t, J= 6.5 Hz), 3.84 (1H, m, J= 4 Hz). Overall yield over four steps, 29%.
Scheme 1.
Larger polyols. Following literature (WENDER et al., p. 13648, 2002), triol was
subjected to PTSA-catalyzed protection with (-)-menthone and then it was
oxidized by Swern protocol. Zinc-promoted Reformatsky reaction (OCAMPO, p. 9325,
2004) of the resulting aldehyde with ethyl bromoacetate, followed by protection
and final LAH reduction, afforded a diastereomeric mixture of 1,3,5,7-
heptanetetraol. This was a preliminary attempt. Refinement of the procedure is
in due course
Computational results. Geometry optimization resulted in polyol dimers connected
via intermolecular hydrogen bonds along with the intramolecular hydrogen bond
for both the diol and triol dimer systems. Two equivalent dimer structures were
found in each case that are connected through concerted mechanism passing
through transition state structure (see Figure 1 for triol).
Figure 1.
The binding energy for the diol and triol dimers were found to be 15.79 and
18.55 kcal/mol respectively. The energy barriers, including zero-point
corrections, for the multiproton transfer were calculated to be 16.39 and 24.36
kcal/mol for the diol and triol respectively.

A. Starting dimer (0.00 Kcal/mol). B. Transition state (16.39 Kal/mol). C. Final dimer (0.00 Kcal/mol)
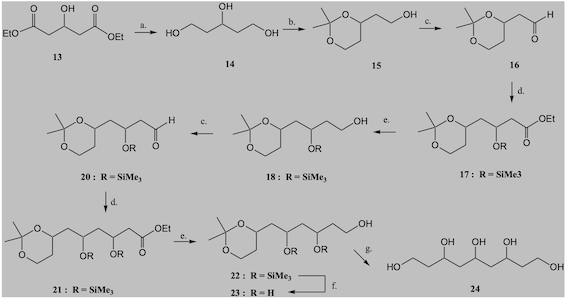
Synthetic route projected for 1,3,5-pentanetriol and larger polyols
Conclusões
Calculations suggest that 1,3-diols or 1,3,5-triols form dimers with binding
energies of 16-19 Kcal/mol. Barriers for multiproton transfers range 16-24
Kcal/mol. With this data, the ability of aliphatic polyols to facilitate
proton transfer reactions will be explored in specific systems including long-
range keto-enol and other tautomerization equilibria, zwitterionic amino acids,
etc. The results will be compared with those which are obtained by using water
chains or wires.
In the mean time, experiments will be attempted with synthesized polyols to prove
the theoretical concept.
Agradecimentos
R.D.Parra acknowledges and thanks DePaul University, USA. R.Ocampo-Cardona and M.
Martinez acknowledge and thank Universidad de Caldas (Vicerrectoria de
Investigaciones y Posgrados) in Colombia
Referências
ABOU-ZIED, O. K., AL-LAWATIA, N., HUSBAND, J., STEINBRECHER, T. Tautomerism in 7-Hydroxyquinoline: a combined experimental and theoretical study in water. J. Phys. Chem. A, 115, 4195-4201, 2011
BLOMBERG, M. R. A., SIEGBAHN, P. E.M. Different types of biological proton transfer reactions studied by quantum chemical methods. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1757 (8), 969-980, 2006
CUKIER, R. I., NOCERA, D. G. Proton-coupled electron transfer. Annu. Rev. Phys. Chem., 49 (1998), pp. 337-369, 1998
FANG, W. H. Ab initio study of the triple-proton-transfer reactions of ground and excited states of 7-hydroxyquinoline in methanol solution. J. Am. Chem. Soc., 120, 7568-7576, 1998
KASS, S. R., SHOKRI, A., ABEDIN, A., FATTAHI, A. Effect of hydrogen bonds on pKa values: importance of networking. J. Am. Chem. Soc., 134, 10646-10650, 2012
LEUTWYLER, S., BACH, A. Water-chain clusters: vibronic spectra of 7-hydroxyquinoline (H2O)n, n=1-4. Chemical Physics Letters, 299, 381–388, 1999
LEUTWYLER, S., BACH, A., MEUWLY, M. Grotthus-type and diffusive proton transfer in 7-hydroxyquinoline (NH3)n clusters. J. Am. Chem. Soc., 123, 11446-11453, 2001
LEUTWYLER, S., BACH, A., TANNER, C., MANCA, C., FREY, H-M. Ground- and excited state proton transfer and tautomerization in 7- hydroxyquinoline (NH3)n clusters: spectroscopic and time resolved investigations. J. Chem. Phys., 119, 5933-5942, 2003
MORI, K., IKUNAKA, M. Synthesis of (-)-talaromycins a and b. Tetrahedron, 43 (1), 45-58, 1987
OCAMPO-CARDONA, R., DOLBIER, W. R. The Reformatsky Reaction in Organic Synthesis. Recent Advances. Tetrahedron, 60 (42), 9325-9374, 2004
PARRA, R. D., SUH, F., RIVERA, V. Diol mediated tautomerization of Glycine: a DFT study. Am. J. Undergrad. Res., 16 (1), 33-39, 2019
QUINTARD, A., SPERANDIO, C., RODRIGUEZ, J. Catalytic strategies towards 1,3-polyol synthesis by enantioselective cascades creating multiple alcohol functions. Organic & Biomolecular Chemistry, 18, 1025-1035, 2020
WENDER, P.A., BARYZA, J. L., BENNETT, C. E., et al. The practical synthesis of a novel and highly potent analog of bryostatin. J. Am. Chem. Soc., 124 (46), 13648-13649, 2002
















