Autores
Bravo P., N.F. (UNIVERSIDAD DE LOS ANDES, COLOMBIA) ; Portilla, J. (UNIVERSIDAD DE LOS ANDES, COLOMBIA) ; Bonacorso, H.G. (UNIVERSIDAD FEDERAL SANTA MARIA) ; Almeida, B. (UNIVERSIDAD FEDERAL SANTA MARIA)
Resumo
Based on the photophysical properties of coumarin and pyrazolo[1,5-a]pyrimidine
(PP) derivatives, was proposed the synthesis of new conjugated fluorophore
systems coumarin - pyrazolo[1,5-a]pyrimidine with different hidrazides receptor.
The UV-vis and fluorescence spectra of the compounds were measured in different
solvents. The absorption spectra of compounds showed a main band between 420–
460 nm that was assigned to an ICT process. Photophysical properties of the
compounds against metal detection were evaluated. Changes in the absorption and
emission were observed for metals such as Cu, Zn and Ni. Although the detection
was not selective towards a single metal, the results obtained will contribute
to the development of future generations of more sensitive and selective
chemosensors.
Palavras chaves
Cumarin; pyrazolo[1,5-a]pirimidine; Photofísical properties
Introdução
The existence of toxic ions in water bodies has harmful impacts on the health of
the world population, (ABDEL et al, p. 67, 2018) therefore, work has been done
on the development of chemosensors for its detection. (LEE et al, p. 5563,
2016). Based on the photophysical properties of coumarin and pyrazolo[1,5-
a]pyrimidine (PP) derivatives, their use for molecular sensor design is favored;
specifically, probes for the detection of dissolved ionic species of biological
and/or environmental impact.
Coumarin derivatives are remarkable fluorophores because they have excellent
physicochemical properties such as high fluorescence quantum yields,
photostability, among ohers (JIAO et al, p. 403, 2017) (YANG et al, p. 212,
2017). Recent studies on the photophysical properties of coumarins show key
structure-ownership relations, which have been essential for the design of
fluorescent probes (BOCHKOV et al, 2017).
On the other hand, some fused aza-heterocyclic compounds containing the pyrazole
ring, such as pyrazolo[1,5-a]pyrimidine (PP) derivatives, stand out for their
synthetic versatility and wide range of physicochemical applications that many
derivatives have (CASTILLO et al, p. 28483, 2017), (TIGREROS et al, p. 395,
2019). This system acts by an ICT fluorescence process, which is favored by
incorporating an electron-donor aryl group at position 7 (CASTILLO et al, p.
10887, 2018) ;however, the fluorescence emission is maintained but weakened with
an electron-acceptor group (TIGREROS et al, p. 39542, 2020).
Therefore, obtaining new pigments with the architectures and studying their
photophysical properties is a great challenge in detection chemistry.
Material e métodos
This section describes the experimental design that will be carried out to
achieve the objectives of the research proposal. The synthetic pathway for the
acceptor molecule fragment involves the formation of 3-formylpyrazolo[1,5-
a]pyrimidine 4 via a three step sequence from acetyl derivatives 1, ie i.
formation of the β-enaminone 2 with DMF-DMA, ii. cyclocondensation with 3-
methyl-5-aminopyrazole 3 and iii. formylation of 4 with POCl3 in DMF.
Subsequently, the reaction was carried out with carbohydrazide, semicarbazide
and acethydrazide in acid medium to have the recognition site in the molecular
sensor. 6 (Scheme 1).
The characterization of the compounds was carried out with structural analysis
methods, such as NMR spectroscopy, high resolution mass spectrometry (HRMS), UV-
vis and fluorescence spectroscopy.
Emission and absorption studies was carried out in different solvents, seeking
to determine the changes with the medium because the molecules will have
different polarities or energies when their ground and excited states are
compared, making them susceptible to changes related to the system
microenvironment.
For the detection of ions, the probe was mixed with different ions and the
optical changes was identified with the naked eye or using a manual lamp with
the long wavelength. Then, the quantitative study was done to determine the
potential of the sensor. Active probes was diluted and then separately add a
free ion equiv of different cations (its nitrates), where the test must also be
carried out with the mixture of all the cations. Fluorescence spectra are
recorded at the excitation wavelength with the highest quantum yield. These
analyzes will be done with the help of the OriginLab program.
Resultado e discussão
Pyrazolo[1,5-a]pyrimidines (PPs) 6a–c were synthesized by a two-step synthesis
sequence starting from 3-acetyl-7-(diethylamino)-2H-chromen-2-one. Compounds
were synthesized in an overall yield of 85–90% with protocols previously
reported in our research group. The UV-vis and fluorescence spectra of the
compounds 6a–c were measured in different solvents (Fig. 1). The absorption and
emission spectra of these PPs are highly dependent on the nature of coumarin at
position 7.
The absorption spectra of 6a–c showed a main band between 420– 460 nm that was
assigned to an intramolecular charge transfer process. In general, PPs
derivatives displayed the same differences in the absorption spectra as a result
of the π-extended conjugation in the coumarin unit. In all cases, the absorption
maximum wavelengths (λabs) remained without noticeable changes, regardless of
the solvent used. Furthermore, the molar absorption coefficients decreased
somewhat with increasing solvent polarity. When R2 changes from -CH3 to –NH-NH2
or –NH2, a decrease in absorption and fluorescence is observed.
When PPs 6a–c were excited at their λabs in solution at 20°C, they exhibited
fluorescence bands at around 525–575 nm (Fig. 1). It is notable that compound 6a
possesses the highest absorption coefficient (ε) and quantum yield (σF).
Similarly, the photophysical properties of the compounds against metal detection
were evaluated and variable results were found. With the absorption spectra,
changes in the coloration and in the spectrum were observed for metals such as
Cu, Zn and Ni. In the emission, the increase in fluorescence or CHEF phenomenon
was demonstrated with the interaction with Zinc, while a CHEQ phenomenon or
fluorescence quenching with Copper and Nickel was evidenced.
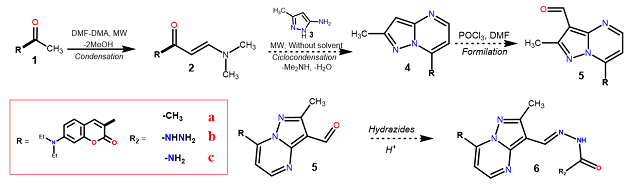
Scheme 1. Synthesis of conjugated coumarin - pyrazolo[1,5-a]pyrimidine with different hidrazide receptor 6
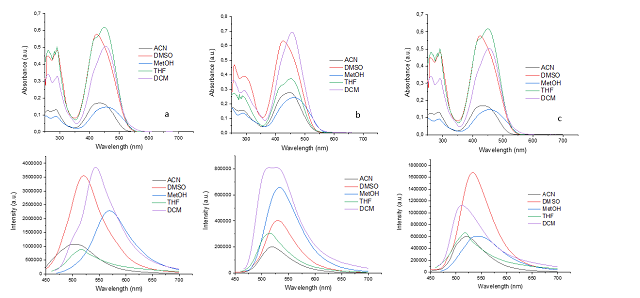
Figure 1. (a) UV–Vis absorption spectra of 6a-c (27,5 µM) in all solvents at room temperature. (b) Emission spectra of 6a-c (5,5 µM) in all solvents.
Conclusões
It was possible to establish that the synthesized compounds with good yields,
detect Zn by fluorescence enhancement or CHEF phenomena, while they detect Ni and
Cu by fluorescence quenching through CHEQ processes.
Although the detection was not selective towards a single metal, the results
obtained will contribute to the development of future generations of more
sensitive and selective chemosensors, where the design of new detection methods
will provide the scientific community with adequate tools to monitor and/or
extract inorganic species pollutants in the environmental context.
Agradecimentos
Chemistry Department and Vicerrectorıa de Investigaciones at the Universidad de
Los Andes. Science faculty (P: INV-2021-126-2326, INV-2022-137-2413). MINCIENCIAS
for a doctoral fellowship (Fund of the General Royalties System)
Referências
1. Abdel, H.; Ahmed, A.; Shaima, E.; Bayaumy, M.; Mona, A. Environ. Nanotechnology, Monitoring & Management. 2018, 9, 67–75.
2. Bochkov, A. Y.; Akchurin, I. O.; Traven, V. F. Heterocycl. Commun., 2017, 23(2).
3. Castillo, J.-C.; Rosero, H.; Portilla, J. RSC Adv. 2017, 7, 28483–28488.
4. Castillo, J.-C.; Tigreros, A.; Portilla, J. J. Org. Chem. 2018, 83, 10887−10897.
5. Jiao, Y.; Zhou, L.; He, H.; Yin, J.; Duan, C. Talanta 2017, 162, 403–407.
6. Lee, S, Bok, K, Kim, J, Kim, S, Kim, C. Tetrahedron 2016, 72, 5563–5570.
7. Tigreros, A.; Rosero, H.; Castillo, J.-C.; Portilla, J. Talanta 2019, 196, 395–401.
8. Tigreros, A., Aranzazu, S., Bravo, N., Zapata, J., Portilla, J.RSC Adv., 2020, 10, 39542–39552
9. Yang, L.; Wang, C.; Chang, G.; Ren, X. Sens. Actuator B-Chem. 2017, 240, 212–219.
















