Autores
Rios, M.C. (UNIVERSIDAD DE LOS ANDES) ; Tigreros, A. (UNIVERSIDAD DE LOS ANDES) ; Aranzazu, L. (UNIVERSIDAD DE LOS ANDES) ; Portilla, J. (UNIVERSIDAD DE LOS ANDES)
Resumo
Five new pyrazolo[1,5-a]pyrimidine fluorescent dyes (2a-f) bearing boron difluoro/ß-diketonate
(dioxaborinine) moiety were successfully synthesized in a one-pot strategy, and their structures were fully
characterized. The main objective of the work was to compare the optical properties of the synthesized
compounds concerning their precursors without the dioxaborinine moiety (1a-f). The optical properties were
studied via UV-VIS, fluorescence spectroscopy, and emission in the solid state. The photophysical properties
of the compounds were compared with that of their starting compounds. The synthesized compounds
showed high emission efficiencies (φ = 0.04 - 0.69) as a result of an ICT process between pyrazolo[1,5-
a]pyrimidine (donor) and dioxaborinine (acceptor).
Palavras chaves
Pyrazolo[1,5-a]pyrimidine; dioxaborinine; fluorescence
Introdução
In recent decades, fluorescent dyes have won the attention of researchers in various fields, and they possess
important applications in many areas such as organic electronics (OLED, OFET)(SALEHI e colab., 2019),
chemical detection(TIGREROS, Alexis e PORTILLA, 2020), creation of fluorescent biomarkers(GAO e colab.,
2021), and as biomaterials in medical diagnostics and photodynamic therapy(MALDONADO-CARMONA e
colab., 2020; SHAN e colab., 2018). In this context, it should be noted that the boron β-diketone difluoride
complexes, also known as dioxaborinine derivatives, have been extensively studied due to their interesting
photophysical properties(COLLOT, 2021) such as large molar absorption coefficients(POLISHCHUK e colab.,
2021), size-dependent luminescence(FEDORENKO e MIROCHNIK e colab., 2021), mechanofluorochromic
behavior(ZHANG e colab., 2018), two-photon absorption activity(JU e colab., 2019), controllable emission
wavelength by polymeric matrices(FEDORENKO e KHREBTOV e colab., 2021) and high fluorescence quantum
yields. These properties allow this type of system to have applications in fluorescent sensing of
amines(SEENIVASAGAPERUMAL e SHANMUGAM, 2018; ZHAI e colab., 2017) or cyanide(TAMILARASAN e
colab., 2020), bioimaging (cells, tissues, and in vivo)(COLLOT, 2021), or electroluminescence devices (OLED)
(KIM e colab., 2018).
Another exciting family of fluorescent compounds is the based on pyrazolo[1,5-]pyrimidine ring, dyes
that have been fascinating in the last few years due to their high fluorescence quantum yields in both solution
and solid-phase and excellent photo and thermostabilities,(TIGREROS, Alexis e ARANZAZU e colab., 2020;
TIGREROS, Alexis e MACÍAS e colab., 2021) which showed great applications mainly as fluorescent
chemosensors(TIGREROS, A. e colab., 2019; TIGREROS, Alexis e CASTILLO e colab., 2020; TIGREROS, Alexis
e colab., 2022; TIGREROS, Alexis e ZAPATA-RIVERA e colab., 2021) or biomarkers(YANG e colab., 2020).
The extension of the π-conjugation and the electronic coupling of different fluorophores like triphenylamine-
fluorene(TIGREROS, A. e colab., 2014), triphenylamine-BODIPY(LEE e colab., 2021), or pyrene-
BODIPY(IRMLER e colab., 2019) has allowed the improvement of the optical properties or induced new ones
such as solvatofluorochromism, Near Infrared II two-photon absorption(LI e colab., 2020), or FRET(PORCU e
colab., 2018). Therefore, combining two or more fluorophores can be a good strategy for designing new
compounds with exciting photophysical properties.
Based on the abovementioned facts, here we report on the synthesis and the photophysical characterization
of a series of pyrazolo[1,5-]pyrimidine–dioxaborinine hybrids, with Friedel-Craft acetylation, followed by
subsequent acetylation as the key synthetic steps in the preparation of the target compounds. Their
photophysical and thermal properties were also studied. Finally, a relationship was made between the
structure (substituents) and the optical and thermostability.
Material e métodos
All starting materials were weighed and handled in air at room temperature. The reactions were monitored by
TLC and visualized by UV (254 nm). All compounds were synthetized via Microwave Assisted Organic
Synthesis using a sealed reaction vessel (10 mL, max pressure = 300 psi) containing a Teflon-coated stirring
bar (obtained from CEM). Microwave-assisted reactions were performed in a CEM Discover focused
microwave (ν = 2.45 GHz) reactor equipped with a built-in pressure measurement sensor and a vertically
focused IR temperature sensor; controlled temperature, power, and time settings were used for all reactions.
Compounds were purified through flash chromatography on silica gel. NMR spectra were recorded at 400
MHz (1H) and 100 MHz (13C) at 298 K. NMR spectroscopic data were recorded in CDCl3 or DMSO. DEPT
spectra were used for the assignment of carbon signals. Chemical shifts (δ) are given in ppm and coupling
constants (J) are given in Hz. The following abbreviations are used for multiplicities: s = singlet, d = doublet, t
= triplet, and m = multiplet. Melting points were collected using a Stuart SMP10 melting point apparatus, and
the acquired data are uncorrected. High-resolution mass spectra (HRMS) were recorded using an Agilent
Technologies Q-TOF 6520 spectrometer by electrospray ionization (ESI). The electronic absorption and
fluorescence emission spectra were recorded in quartz cuvettes having a path length of 1 cm. UV–vis and
emission measurements were achieved at room temperature (20 °C). For fluorescence measurements, both
the excitation and the emission slit widths were 5 nm.
Resultado e discussão
1. Synthesis of compounds 2a-f
The synthetic route for compounds 2a-f is shown in Scheme 1. The pyrazolo[1,5-a]pyrimidine starting
materials 1a-f were obtained according to previous procedures. The synthesis of compounds 2a-f was
accomplished by using BF3OEt2 and acetic anhydride in DCE solution at 90ºC for 24h in good yields (49-70
%) in a one-pot strategy. First, an electrophilic aromatic substitution reaction occurs to afford acetylated
intermediates. Next, a second acetylation reaction occurs in the acetyl group due to the excess of acylating
agent (Ac2O-BF3OEt2). Finally, the formation of boron difluoro β-diketonate 2 is obtained in the presence of
BF3OEt2 as reported before by many authors(NAGAI e colab., 2008).
Absorption and emission properties
The UV-vis absorption and fluorescence spectroscopic data in THF solution and emission in the solid-state of
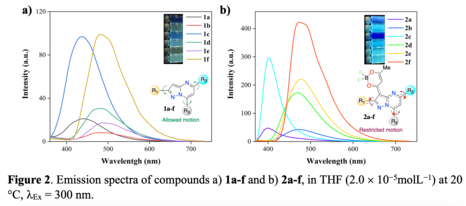
compounds 1a-f and 2a-f are shown in Figures 1 and 2 and the corresponding photophysical properties are
summarized in Table 1.
Compounds 1a-f exhibit the typical dual-bands spectra of pyrazolo[1,5-a]pyrimidine derivates observed in the
ranges of 300-400 nm and 400-550 nm, corresponding to the π-π* and n-π* and, in less extension, to an
intramolecular charge transfer (ICT) transition between the pyrazole (donor) and pyrimidine (acceptor)
units(TIGREROS, Alexis e ARANZAZU e colab., 2020). As expected, the absorption coefficient values (ε)
increase with the sequential introduction of phenyl groups in the pyrazolo[1,5-a]pyrimidine core.
On the other hand, the introduction of electron-withdrawing group (dioxaborine) results in a slight red shift of
the absorption band of less energy with an important increase of the absorption coefficient in compounds
2a-f. Therefore, the good electronic communication between two units is evident. However, the ICT process is
slightly enhanced after a good acceptor group was incorporated, reflecting the low electron donating
capability of the pyrazole unit when compared with other electron donating groups (e. g., triphenylamine: ICT
band λ= 425 nm)(TAMILARASAN e colab., 2020).
In general, as the number of phenyl groups increase the absorption coefficient of the ICT transition decrease.
Fascinatingly, the highest ε value was found in compound 2a (37378 Lmol-1cm-1) which has the less π-
conjugation. Therefore, the ICT transition is the main responsible of the absorption spectrum. Thanks to the
high coplanarity between aryl groups and pyrazole moiety(PORTILLA e colab., 2005, 2007), good absorption
coefficient values were observed in compound 2c. However, compounds with phenyl groups at position 7 (2b
and 2e) display the lower ε values for the ICT bands. Meanwhile, as expected compounds with phenyl groups
at position 2 and 7 (2d and 2f) display intermediate values. In summary, the tendency found (2a > 2c > 2d >
2f > 2b > 2e) indicates that substitution pattern and the electronic communication with the periphery rings is
critical for the absorption capability.
When excited at 300 nm, derivatives 1a-f exhibited fluorescence emission in the blue-green region (441–491
nm), where the number and position of phenyl substituents did not play an important role in the excited state
energy of these fluorophores, Figure 2a. Meanwhile, the efficiency of the emission (φ) ranges from 0.03 (1a)
to 0.30 (1f). The coplanarity expected between phenyl groups and pyrazole moiety at position 2 in
compounds 1c, 1d and 1f could explain the better quantum yield observed in these cases.
Probes 2a-f exhibited fluorescence emission in the blue-green region (400-479 nm) under excitation at λ =
300 nm, Figure 2b. As already observed with compounds 1a-f, for these compounds, the number and position
of phenyl substituents present on the pyrazolo[1,5-a]pyrimidine unit slightly affect the fluorescence maximum
location. Furthermore, compounds 2a-f show a considerable increase in their fluorescence quantum yield in
THF compared to 1a-f, with φ values as high as 0.69 for 2f. Thus, the extension of the π-conjugation and the
ICT character of the transition cause by a dioxaborinine moiety significantly affect the emission from the
excite state in solution. These results can be explained by the restricted motion in 2a-f as a result of ICT
process, avoiding the non-radiative process due to rotation of phenyl groups in compounds 1a-f(XU e colab.,
2022).
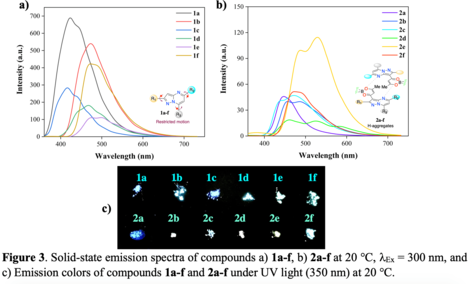
3. Solid-phase emission properties
Figure 3a shows the results from the photophysics in the solid-state for compounds 1a-f, where the samples
were treated as a powder. The solid-phase slightly affects the emission maximum location in substituted
pyrazolo[1,5-a]pyrimidine from 417 nm (1c) to 488 nm (1e). The emission efficiency of these compounds
appeared to be quite different from those observed in solution with values as high as 0.51 (1a)
The introduction of dioxaborinine moiety, except by 2e λEm = 530 nm, did not affect substantially the
emission maximum location (448 nm in 2a to 480 nm in 2f). The emission behavior in 2e could be ascribed to
a micro-crystalline disposition that induces a better donor-acceptor interaction as reported before in
pyrazolo[1,5-a]pyrimidine with nitrobenzene as a substituent group.(TIGREROS, Alexis e colab., 2022)
Importantly, compounds containing dioxaborinine unit 2a-f show a considerable decrease in their
fluorescence quantum yield at solid-phase when compared to solutions in THF, with quantum yields as low as
0.02 for 2a and 2d. Therefore, these results indicate that the dioxaborinine moiety afforded higher
interactions such as π-stacking between the heterocyclic rings present in the hybrid chemical structures.
Those interactions could deactivate the excited state and decrease the quantum yield through the formation
of H-aggregates(WITTE e colab., 2021).
Conclusões
In conclusion, we have synthesized a set of pyrazolo[1,5-a]pyrimidine-dioxaborinine hybrid compounds 2a-f
in a one-pot manner using Friedel-Craft acylation conditions as the key step and using easily available
pyrazolo[1,5-a]pyrimidine 1a-f as starting substrates. From results obtained, the best candidates for optical
applications in solid-phase such as OLED or solid-state laser are dioxaborinine-free pyrazolo[1,5-
a]pyrimidines 1a-f. Meanwhile, those applications requiring high fluorescence intensities like sensing or
bioimages may use derivates 2a-f which display quantum yields as high as 0.69. The relatively easy
availability by synthesis together with favorable photophysical properties make some of these compounds
promising candidates for future application in optical devices, sensing or bioimages.
Agradecimentos
The authors wish to thank Universidad de los Andes for financial support.
Referências
COLLOT, Mayeul. Recent advances in dioxaborine-based fluorescent materials for bioimaging applications. Materials Horizons, v. 8, n. 2, p. 501–514, 2021.
FEDORENKO, Elena V e KHREBTOV, Aleksandr A e colab. Polymer films doped with dimethylaminostyryl β-diketonates of boron difluoride: Spectral properties and influence of the polymer matrix. Journal of Luminescence, v. 235, p. 118043, 2021.
FEDORENKO, Elena V e MIROCHNIK, Anatolii G e KARPENKO, Aleksander A. Size-dependent luminescence of boron difluoride 1-(2′-naphthyl)butanedionate-1,3. Journal of Photochemistry and Photobiology A: Chemistry, v. 420, p. 113508, 2021.
GAO, Liqian e colab. Fluorescent probes for bioimaging of potential biomarkers in Parkinson’s disease. Chemical Society Reviews, v. 50, n. 2, p. 1219–1250, 2021.
IRMLER, Peter e colab. Four different emissions from a Pt(Bodipy)(PEt3)2(S-Pyrene) dyad. Dalton Transactions, v. 48, n. 4, p. 1171–1174, 2019.
JU, Chenggong e colab. Solvent dependent linear and nonlinear optical properties of triphenylamine unit incorporated difluoroboron β-diketonate complexes. Dyes and Pigments, v. 162, p. 776–785, 2019.
KIM, Dae-Hyeon e colab. High-efficiency electroluminescence and amplified spontaneous emission from a thermally activated delayed fluorescent near-infrared emitter. Nature Photonics, v. 12, n. 2, p. 98–104, 2018.
LEE, Jae moon e colab. Mechanofluorochromism of Triphenylamine-BODIPY: Effect of twisted intramolecular charge transfer and restriction in rotation on fluorescence. Dyes and Pigments, v. 185, p. 108864, 2021.
LI, Benhao e ZHAO, Mengyao e ZHANG, Fan. Rational Design of Near-Infrared-II Organic Molecular Dyes for Bioimaging and Biosensing. ACS Materials Letters, v. 2, n. 8, p. 905–917, Ago 2020.
MALDONADO-CARMONA, Nidia e colab. Conjugating biomaterials with photosensitizers: advances and perspectives for photodynamic antimicrobial chemotherapy. Photochemical & Photobiological Sciences, v. 19, n. 4, p. 445–461, 2020.
NAGAI, Atsushi e colab. Highly Intense Fluorescent Diarylboron Diketonate. The Journal of Organic Chemistry, v. 73, n. 21, p. 8605–8607, Nov 2008.
POLISHCHUK, Vladyslav e colab. Highly Fluorescent Dianionic Polymethines with a 1,3,2-Dioxaborine Core. The Journal of Organic Chemistry, v. 86, n. 7, p. 5227–5233, Abr 2021.
PORCU, Pasquale e colab. Design of Novel Pyrene-Bodipy Dyads: Synthesis, Characterization, Optical Properties, and FRET Studies. Molecules . [S.l: s.n.]. , 2018
PORTILLA, Jaime e colab. 7-Amino-2-tert-butyl-5-methylpyrazolo[1,5-a]pyrimidine: a three-dimensional framework structure built from two NH...N hydrogen bonds. Acta Crystallographica Section C, v. 63, n. 1, p. 26–28, Jan 2007.
PORTILLA, Jaime e colab. Hydrogen-bonded chains in isostructural 5-methyl-2-(4-methylphenyl)-7,8-dihydro-6H-cyclopenta[g]pyrazolo[1,5-a]pyrimidine, 2-(4-chlorophenyl)-5-methyl-7,8-dihydro-6H-cyclopenta[g]pyrazolo[1,5-a]pyrimidine and 2-(4-bromophenyl)-5-methyl-7. Acta Crystallographica Section C, v. 61, n. 7, p. 452–456, Jul 2005.
SALEHI, Amin e colab. Recent Advances in OLED Optical Design. Advanced Functional Materials, v. 29, n. 15, p. 1808803, Abr 2019.
SEENIVASAGAPERUMAL, Sriram Babu e SHANMUGAM, Sivakumar. Fluorescent β-ketothiolester boron complex: substitution based “turn-off” or “ratiometric” sensor for diamine. New Journal of Chemistry, v. 42, n. 5, p. 3394–3400, 2018.
SHAN, Dingying e colab. Polymeric biomaterials for biophotonic applications. Bioactive Materials, v. 3, n. 4, p. 434–445, 2018.
TAMILARASAN, Duraiyarasu e colab. Reversible Addition of Cyanide to Triphenylamine Attached Difluoroboron β-Diketonate Facilitated Selective Colorimetric and Fluorimetric Detection of Cyanide Ion. European Journal of Organic Chemistry, v. 2020, n. 8, p. 993–1000, 2020.
TIGREROS, A. e colab. Integrated pyrazolo[1,5-a]pyrimidine–hemicyanine system as a colorimetric and fluorometric chemosensor for cyanide recognition in water. Talanta, v. 196, p. 395–401, 2019.
TIGREROS, A. e ORTIZ, A. e INSUASTY, B. Effect of π-conjugated linkage on photophysical properties: Acetylene linker as the better connection group for highly solvatochromic probes. Dyes and Pigments, v. 111, p. 45–51, 2014.
TIGREROS, Alexis e ARANZAZU, Sandra-L e colab. Pyrazolo[1,5-a]pyrimidines based fluorophores: A comprehensive theoretical-experimental study. RSC Advances, v. 10, p. 39542–39552, 2020.
TIGREROS, Alexis e CASTILLO, Juan-Carlos e PORTILLA, Jaime. Cyanide chemosensors based on 3-dicyanovinylpyrazolo[1,5-a]pyrimidines: Effects of peripheral 4-anisyl group substitution on the photophysical properties. Talanta, v. 215, p. 120905, 2020.
TIGREROS, Alexis e MACÍAS, Mario e PORTILLA, Jaime. Expeditious ethanol quantification present in hydrocarbons and distilled spirits: Extending photophysical usages of the pyrazolo[1,5-a]pyrimidines. Dyes and Pigments, v. 202, p. 110299, 2022.
TIGREROS, Alexis e MACÍAS, Mario e PORTILLA, Jaime. Photophysical and crystallographic study of three integrated pyrazolo[1,5-a]pyrimidine–triphenylamine systems. Dyes and Pigments, v. 184, p. 108730, 2021.
TIGREROS, Alexis e PORTILLA, Jaime. Recent progress in chemosensors based on pyrazole derivatives. RSC Advances. [S.l.]: Royal Society of Chemistry. Disponível em: <https://pubs.rsc.org/en/content/articlehtml/2020/ra/d0ra02394a>. Acesso em: 18 abr 2021. , 22 Maio 2020
TIGREROS, Alexis e ZAPATA-RIVERA, Jhon e PORTILLA, Jaime. Pyrazolo[1,5-a]pyrimidinium Salts for Cyanide Sensing: A Performance and Sustainability Study of the Probes. ACS Sustainable Chemistry & Engineering, v. 9, n. 36, p. 12058–12069, Set 2021.
WITTE, Felix e colab. Fluorescence Quenching in J-Aggregates through the Formation of Unusual Metastable Dimers. The Journal of Physical Chemistry B, v. 125, n. 17, p. 4438–4446, Maio 2021.
XU, Changhuo e colab. Molecular Motion and Nonradiative Decay: Towards Efficient Photothermal and Photoacoustic Systems. Angewandte Chemie International Edition, v. n/a, n. n/a, p. e202204604, Maio 2022.
YANG, Xiu-Zhi e colab. The application of bioactive pyrazolopyrimidine unit for the construction of fluorescent biomarkers. Dyes and Pigments, v. 173, p. 107878, Fev 2020.
ZHAI, Lu e colab. New non-traditional organogelator of β-diketone-boron difluoride complexes with terminal tetraphenylethene: Self-assembling and fluorescent sensory properties towards amines. Dyes and Pigments, v. 145, p. 54–62, 2017.
ZHANG, Ling e WANG, Xin e ZHAO, Xiong-Yan. The reversible mechanofluorochromic property of an asymmetric diketonate boron complex at room temperature. Journal of Luminescence, v. 202, p. 420–426, 2018.
















