Autores
dos Santos Filho, J.M. (UFPE) ; de Souza Castro, M.V.B. (UFPE)
Resumo
The pursuit of new biological active and structurally diverse molecules is the
main core of medicinal chemistry, which can be achieved by means of different
theoretical and experimental strategies, including molecular simplification.
Starting from a structural model of more complexity, it is possible to design
new molecules with easier synthetical requirements, improved physicochemical
properties, and even new biological profiles. Based on this strategy, 10
ferrocenyl-N-acylhydrazone (Fc-NAH) hybrids with potential biological
activity have been planned, synthesized, and characterized using spectroscopic
techniques. The applied synthetical method has led to the stereoselectivity of
the E-isomers, avoiding diastereomeric mixtures that can affect
biological studies.
Palavras chaves
molecular simplification; [i]N[/i]-acylhydrazones; ferrocene
Introdução
Ferrocene (Fc) or di(η5-cyclopentadienyl) iron (II) was
serendipitously discovered by Peter L. Pauson and his graduate student Tom Kealy
in 1951 when they attempted the reductive coupling of the Grignard reagent
cyclopentadienyl magnesium bromide in the presence of ferric chloride. Its
structure was simultaneously proposed by Wilkson and Fischer in 1952 and later
confirmed by X-ray crystallography in 1956. Due to its remarkable chemical
stability, ease of functionalization via electrophilic substitution, redox
properties, high electronic density, and aromaticity, as well as non-toxic
profile and relative lipophilicity, the ferrocenyl moiety has found applications
in several fields of chemistry, especially medicinal chemistry [ŠTĚPNIČKA,
2022]. In fact, the association of the ferrocene scaffold with recognized
pharmacophoric structures has led to important improvements in the biological
activities of resulting molecules, confirming its importance for medicinal
chemistry [SINGH et al, 2019].
Indeed, the N-acyl hydrazone (NAH) scaffold is, just like ferrocene,
associated with a plethora of biological activities, and has been recognized as
a privileged structure in medicinal chemistry [VERMA et al, 2014].
Supported by this
evidence, the molecular hybridization strategy was exploited in our research
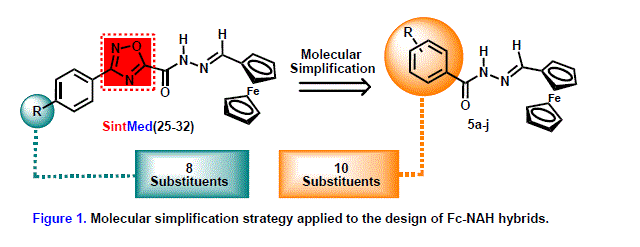
group to design and synthesize a series of ferrocene-N-acyl hydrazone-
1,2,4-oxadiazole derivatives SintMed(25-32) (Figure 1) for the investigation of
their antimalarial activities [DOS SANTOS FILHO et al, 2016]. Despite its
important
role in chemical and biological behavior, the 1,2,4-oxadiazole ring construction
requires more synthetic steps, which imposes some restrictions on the molecular
diversity of the series, which is usually essential to the discovery of new lead
structures or even new drugs.
Analyzing the structure of derivatives SintMed(25-32), another usual strategy
applied in medicinal chemistry, the molecular simplification [WANG et al,
2019], seems
to be a natural and promising approach for the discovery of new bioactive
molecules. The removal of the 1,2,4-oxadiazole ring leads to smaller
molecules, whose preparation could be easier, faster, and less expensive could
disclose a larger diversity of structures, which can potentialize the discovery
of biologically active products, especially considering that the simplified
structures retain the important N-acyl hydrazone moiety (Figure 1).
Therefore, 10 ferrocenyl-N-acyl hydrazone (Fc-NAH) hybrids have been
planned, synthesized, fully characterized, and are expected to bring to light
new effective biological active compounds, as well as new lead structures for
further investigations in this field.
Material e métodos
Reactions’ progress was monitored by thin-layer chromatography (TLC), performed
onto glass-backed plates of silica gel 60 F254 with gypsum from Merck, and all
compounds were detected by ultraviolet light (254 nm). Melting points were
determined with a capillary apparatus and are uncorrected.
NMR spectra were recorded at 400 MHz for hydrogen and 100 MHz for carbon.
Analyses were determined in DMSO-d6 with chemical shift values (δ) in
parts per
million (ppm). Infrared (IR) spectra were recorded on a Tensor27 FTIR
spectrometer from
Bruker with the samples being analyzed as KBr pellets.
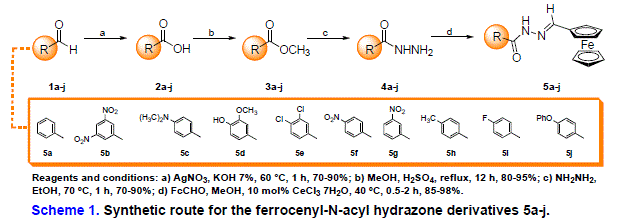
The synthetical route is depicted in Scheme 1 and starts with the oxidation of
aldehydes 1a-j using silver nitrate and potassium hydroxide aqueous solution
(7%) at 60°C for 1 h, leading to the corresponding carboxylic acids 2a-j.
Fischer esterification of 2a-j was carried out in presence of methanol and
sulfuric acid as catalyst so that the methyl esters 3a-j could be obtained in
good yields. Conversion of 3a-j into hydrazides 4a-j was easily performed by
reaction with hydrazine hydrate under reflux. The hydrazides 4a-j were the key
intermediates for the synthesis of the ferrocene-N-acyl hydrazones 5a-j,
which was carried out under mild conditions in the presence of
CeCl3·H2O and methanol at 40°C during 10-30 minutes. After
filtration, the crude products were found to be pure and could be characterized
using spectroscopic techniques.
Resultado e discussão
The design of the Fc-NAH derivatives was based on the molecular simplification
of the ferrocene-N-acyl hydrazone-1,2,4-oxadiazoles SintMed(25-32) by
removing the heterocyclic ring. This strategy has allowed the synthesis of 10
novel ferrocene-N-acyl hydrazone hybrids 5a-j (Figure 1) with new
physicochemical properties.
The synthetic route to prepare the planned ferrocene-N-acyl hydrazones
5a-
j, depicted in Scheme 1, has started with the silver (I)-mediated oxidation of
commercially available aldehydes 1a-j under basic conditions, leading to the
corresponding carboxylic acids 2a-j as solid materials with good yields. By
using
Fischer esterification, methyl esters 3a-j were easily obtained, and thus
converted into the corresponding hydrazides 4a-j under reflux in the presence of
hydrazine hydrate in excess. Finally, the reaction between the hydrazide
derivatives 4a-j and ferrocene carboxaldehyde was carried out in the presence of
cerium (III) chloride heptahydrate (CeCl3·7H2O) as a
catalyst, following a method developed in our laboratory [dos Santos Filho,
2014] with good to excellent isolated yields (85-98%) of the Fc-NAH hybrids, and
high purity of crude products.
The derivatives 5a-j were characterized by usual spectroscopic techniques,
namely IR, 1H NMR, and 13C NMR, with structural
assignments assisted by DEPT, HSQC, and HMQC experiments, confirming the
structures and stereochemical features for each compound. 1H NMR
analysis of crude Fc-NAH derivatives revealed that only the E-isomer was
formed, in agreement with previously reported outcomes for other compounds
synthesized using this method [DOS SANTOS FILHO, 2014]. Some preliminary data
are given for each compound.
5a: Rf 0.50 (AcOEt/Hexanes 1:1), red powder, yield 93%, mp
178.8-180.5°C (from dioxane/H2O 1:1);
5b: Rf 0.57 (AcOEt/Hexanes 1:1), red powder, yield 94%, mp
248.2-250.5°C (from dioxane/H2O 1:1);
5c: Rf 0.30 (AcOEt/Hexanes 1:1), orange powder, yield 86%, mp
232.6-234.2°C (from dioxane/H2O 1:1);
5d: Rf 0.10 (AcOEt/Hexanes 1:1), red powder, yield 94%, mp
158.5-160.3°C (from dioxane/H2O 1:1);
5e: Rf 0.72 (AcOEt/Hexanes 1:1), red powder, yield 98%, mp
122.3-124.1°C (from dioxane/H2O 1:1);
5f: Rf 0.58 (AcOEt/Hexanes 1:1), red powder, yield 96%, mp
236.3-238.1°C (from dioxane/H2O 1:1);
5g: Rf 0.58 (AcOEt/Hexanes 1:1), red powder, yield 97%, mp
224.6-226.5°C (from dioxane/H2O 1:1);
5h: Rf 0.49 (AcOEt/Hexanes 1:1), red powder, yield 95%, mp
244.0-244.4°C (from dioxane/H2O 1:1);
5i: Rf 0.74 (AcOEt/Hexanes 1:1), red powder, yield 85%, mp
205.6-206.9°C (from dioxane/H2O 1:1);
5j: Rf 0.62 (AcOEt/Hexanes 1:1), red powder, yield 86%, mp
128.8-130.7°C (from dioxane/H2O 1:1).
Conclusões
Herein a successful strategy of structural simplification was applied in the
design of a series of novel ferrocenyl-N-acyl hydrazones (Fc-NAH), starting
from a more complex structural leitmotiv. Based on a more simple and
efficient synthetic route, 10 Fc-NAH were readily prepared and fully
characterized by analytical and spectroscopic techniques. The applied synthetic
methodology was stereoselective, leading exclusively to the E-isomers, an
important structural feature, especially due to the perspective of further
biological studies.
Agradecimentos
The authors are grateful to Mrs. Eliete de Fátima V. B. N. da Silva and the
Analytical Centre of Fundamental Chemistry Department, Universidade Federal de
Pernambuco, for the NMR, and IR experiments.
Referências
DOS SANTOS FILHO, J. M. Mild, Stereoselective, and Highly Efficient Synthesis of N-Acylhydrazones Mediated by CeCl3·7H2O in a Broad Range of Solvents, Eur. J. Org. Chem. 29 (2014) 6411-6417.
DOS SANTOS FILHO, J.M., QUEIROZ E SILVA, D.M.A., MACEDO, T.S., TEIXEIRA, H.M.P., MOREIRA, D.R.M., CHALLAL, S., WOLFENDER, J., QUEIROZ, E.F., SOARES, M.B.P., Conjugation of N-acylhydrazone and 1,2,4-oxadiazole leads to the identification of active antimalarial agents, Bioorg Med Chem. 24 (22) (2016) 5693-5701.
SINGH, A., LUMB, I., MEHRA, V., KUMAR, V. Ferrocene-appended pharmacophores: an exciting approach for modulating the biological potential of organic scaffolds, Dalton Trans. 48 (2019) 2840-2860.
ŠTĚPNIČKA, P. Forever young: the first seventy years of ferrocene, Dalton Trans. 51 (2022) 8085-8102.
VERMA, G., MARELLA, A., SHAQUIQUZZAMAN, M., AKHTAR, M., ALI, M.R., ALAM, M.M., A review exploring biological activities of hydrazones, J. Pharm. Bioallied. Sci. 6 (2) (2014), 69-80.
WANG, S., DONG, G., SHENG, C., Structural simplification: an efficient strategy in lead optimization, Acta Pharm. Sin. B, 9 (5) (2019) 880-901.
















